12 Chapter 12. Microbial Biotechnology in Agriculture
Suhaib Ahmad and Abdul Latif Khan
CHAPTER OUTLINE
12.1 Plant Growth and Challenges to Agriculture
12.2 Microbial utilization in agriculture development
12.3 Microbial Biotechnology in Crop Improvement
Learning Objectives
By the end of this chapter, students should be able to:
- Describe how microorganisms support plant growth and stress resistance
- Explain the role of biofertilizers, PGPR, and biocontrol agents in agriculture
- Understand microbial applications in genetic engineering and crop improvement
- Evaluate case studies of GM crops and their impact on sustainable farming
12.1: Plant growth and challenges to agriculture
12.1.1 The Role of Plants and Agriculture
Plants are products of domestication, with gradual long-term changes in their qualitative and quantitative traits because of continuous natural and human-directed selection. Whereas some of the first improvements in plants and animals could have resulted from chance alone, the eyes and brains of the “primitive” scientist-farmer were crucial in selecting the good from the bad and the productive and quality crop from the less worthy. Thus, advancements in agriculture and plant science are primarily the result of scientific discoveries, judgments, and historical innovations and are sometimes revolutionary, such as food products, the use of inorganic fertilizers, and, more recently, plant genetic transformation.
Man-made technologies and biotechnologies, including food and fiber, have shaped human life since immemorial. Fermented plant and animal products (e.g., bread, cheese, and wine), conventional crop breeding since the birth of agricultural communities, the “Green Revolution” of later years, molecular marker-assisted selection, and recombinant DNA techniques are biotechnologies. The urgent need to look for alternative biotechnologies and the actual accelerated rate of adopting plant molecular biotechnologies since the breakthrough report of the first transgenic plant is due to four major causes:
1. Increase in world population and the need for more food
2. Recognition that human health is affected by disease-causing pathogenic organisms and by the nutritional quality of foods, especially vitamins and minerals
3. Adverse global climatic changes accompanied by detrimental biotic and abiotic hazards (stresses) to crops and ecosystems
4. Human societies search for novel, non-food plant products such as biomaterials, therapeutics, and biofuels.
Thus, agricultural and plant biotechnologies must be swiftly implemented where population growth is outstripping food production (both for quantity and quality).
12.1.2 Climate Change: A Debacle Against Sustainable Plant Biomass Production
Climate changes (increasing or decreasing temperature, lack or abundance of water) and changes in the chemical composition of terrestrial and aquatic ecosystems have hindered the desired natural productivity of plants and threatened food security (Ahmad & Prasad, 2011). For example, higher temperature stress has been recognized as a significant limiting factor affecting plant growth but has also influenced soil rainfall patterns and moisture flux. It has been estimated that an increase of 3°C to 4°C would cause a reduction in plant productivity by up to 15 to 35% by the end of the 21st century (Tayade et al., 2018). Various types of abiotic stress (flooding, salinity, and heavy metals) have been estimated to reduce plant productivity by 51–82% (Cooke & Leishman, 2016; Mittler & Blumwald, 2010). With the increasing human population, food security can be easily disrupted. Among these stress factors, flooding has been considered a major limiting factor for plant growth and production (Nanjo et al., 2014). Climate-induced changes in the precipitation pattern (Sasidharan et al., 2017) and increased rate of submergence create excessive hypoxia (Lee et al., 2011), and subsidiary stresses such as pathogenesis and herbivory (Hsu & Shih, 2013). Continuous submergence can influence soil nutrient balance, leading to high salinity and/or alkalinity, where the plant utilizes a higher amount of energy to defend its existence (Valliyodan et al., 2016).
According to one estimate, up to 831×106 ha of the earth’s land is saline. Of that, 434×106 ha is affected by soil alkalinity in more than 100 countries, causing severe damage to plant growth and loss of agricultural productivity (Jin et al., 2006; Xu et al., 2013). Alkaline stress hinders plant growth compared with salinity stress (Guo et al., 2010). Despite this, the tolerance mechanisms of plants in response to alkaline stress have received less attention than the adaptive mechanisms of the salinity stress (Degenhardt et al., 2000; Hurkman, 1992; Yang et al., 2008; Zhu, 2001). Plant molecular response pattern to abiotic stress triggers the gene expression profile and biosynthetic pathways and enables signal transduction to produce biochemical metabolites and enzymes that increase the defense responses of plants (Ahuja et al., 2010; Godoy et al., 2021; Razi & Muneer, 2021). However, these adaptive mechanisms at molecular, biochemical, and metabolite levels are variable across different species of plants, their growth conditions, and exposure to stress factors.
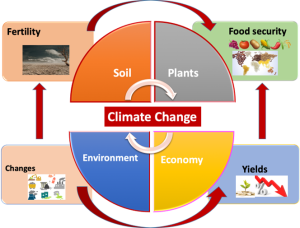
Figure 11.1. Climate change affects plants and health, influencing yield and food security.
12.1.3 Agriculture Crop’s Response to Climatic Change
Physiologically, ESF initiates oxidative stress by producing reactive oxygen species (ROS), superoxide (O2–), singlet oxygen (1O2), hydrogen peroxide (H2O2) that damages functional proteins, lipids, carbohydrates, and nucleic acid (Boyarshinov & Asafova, 2011) (Boogar et al., 2014). Resulting in cellular and tissues injuries are regulated by the activation of a defense mechanism by the production of antioxidant enzymes such as superoxide dismutase (SOD) (Tunc-Ozdemir et al.), peroxidase (PPO), ascorbate peroxidase (APX), polyphenol oxidase (POD), and catalase (CAT) (Cui et al., 2017; Wang et al., 2016) and osmolytes (proline, trehalose, and polyphenols (Sharma et al., 2019). Maintaining a high antioxidant capability to scavenge toxic ROS molecules is associated with enhanced plant tolerance to harsh conditions (Chen et al., 2011; Zaefyzadeh et al., 2009). Reoxygenation during flooding further enhances the post-submergence damage (Fukao et al., 2011). For example, exposure to atmospheric oxygen after 7–10 days of submergence also induced leaf dehydration in rice (Fukao et al., 2011; Setter et al., 2010). Flooding increased ethylene accumulation and a drop in ABA level in AR primordia tissue. ABA treatment inhibited activation of AR primordia by flooding and blocking ABA to reactivate AR primordia in the absence of flooding (Dawood et al., 2016).
One of the significant submergence tolerance regulators in silicious plants, e.g., rice, is SUB1A, which confers tolerance to oxidative stress and dehydration through activation of ROS detoxification and abscisic acid (ABA) responsiveness (Fukao et al., 2011). SUB1A is the master regulator of submergence tolerance, allowing plants to endure complete submergence for 14–16 days (Fukao et al., 2006). Ethylene response factors (ERF-VIIs) have been found to regulate several gene expressions to low oxygen and flooding (Laurentius & Julia, 2015). For example, Five ERF-VII genes (RAP2.12, RAP2.2, RAP2.3, HRE1, and HRE2) are critical regulators for flooding and low-oxygen tolerance in Arabidopsis (Bui et al., 2015; Gasch et al., 2016). Other rice genes, such as ERF-VII, SNORKEL1 and SNORKEL2, have been explicitly found in deep water rice (Hattori et al., 2009). The importance of ERF-VIIs in flooding responses and tolerance is also indicated in Rumex and Rorippa, dicot species from flood-prone environments (van Veen et al., 2014). The hypoxia-induced group-VII ERFs promote adventitious root (AR) elongation, while ethylene inhibits the adventitious root formation (Eysholdt‐Derzsó & Sauter, 2019).
Similarly, high rhizosphere pH significantly affects plant phenotype and genotype under alkaline conditions. Recent studies described the mechanism of alkaline soil tolerance, focusing on the ability of plants to acidify the rhizosphere via plasma membrane H+-ATPase-mediated proton secretion (Fuglsang et al., 2007; Li et al., 2015; Xu et al., 2012; Xu et al., 2013; Yang et al., 2010). Several factors are known to regulate the activity of H+-ATPase. For example, DNAJ HOMOLOG3 (J3) and PROTEIN KINASE5 (PKS5) play critical roles in proton secretion by regulating the interaction between 14-3-3 proteins and the plant plasma membrane H+-ATPase (Fuglsang et al., 2007; Yang et al., 2010). Another study reported that PIN-FORMED2 (PIN2, an auxin efflux transporter) is required to tolerate alkaline stress conditions by regulating proton efflux in the roots (Xu et al., 2012). However, other adaptive mechanisms by those plants that can tolerate alkaline stress and how microbial symbionts can intervene have yet to be explored. Overall, genes involved in cell-wall modification, calcium signaling, ethylene, and reactive oxygen species (ROS) change during cell division and regeneration under conditions of low oxygen (Rajhi et al., 2011; Yamauchi et al., 2018). However, these concurrent molecular signaling and the role of genomics level responses have seldomly been studied and known across economically important plants during ESF and microbiome functions.
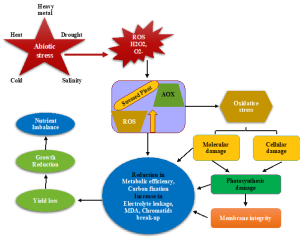
Figure 11.2. Plants exposed to abiotic stress conditions and cellular responses to growth and development.
12.2: Microbial utilization in agriculture development
Introduction: Microbes as Products in Agriculture (Biofertilizers, Biocontrol, and PGPR)
In agriculture, microorganisms are not just passive inhabitants of the soil, they are active partners that shape plant health and productivity. Farmers and scientists have long harnessed microbes such as biofertilizers, biocontrol agents, and plant growth promoting rhizobacteria (PGPR). The following sections explore each category in detail.
Biocontrol:
Biocontrol refers to the use of microbes to protect plants from pests that cause significant damage. Such pests include other pathogenic microbes, fungi, nematodes, and insect larvae. The mechanisms by which beneficial microbes suppress pests are diverse: they may compete for nutrients and ecological niches, produce antibiotics and other chemical compounds that directly inhibit pathogens, or stimulate the plant’s own defense systems through induced resistance (Compant et al., 2005).
A wide range of microorganisms has been employed in biocontrol, but bacteria dominate this field. For example, Pseudomonas species can suppress fungal pathogens and competing bacteria; Bacillus species are well known for their ability to target fungi and insect larvae; while Streptomyces not only inhibit fungi and bacteria but also produce insecticidal metabolites (Choudhary & Johri, 2009). Beyond bacteria, fungi such as Trichoderma are effective against root knot nematodes that damage crops like soybean, peanut, pepper, and tomato. Viruses and protozoa have also been explored for their potential in biocontrol strategies.
Some notable examples illustrate the value of microbial biocontrol. Pseudomonas fluorescens produces antifungal agents that protect plant roots, while Bacillus thuringiensis (Bt) is renowned for its insecticidal properties. Bt is a Gram-positive bacterium that produces toxic crystalline (Cry) proteins during sporulation. These proteins specifically target the gut lining of lepidopteran larvae, leading to death of pests such as corn borer, cotton bollworm, tobacco hornworm, and gypsy moth caterpillars (Bravo et al., 2011). Historically, Bt was first identified as a natural pathogen of silkworms. The genes encoding Cry proteins are plasmid-borne, enabling their transfer and utilization in agricultural biotechnology.
Over the past century, Bt toxins have been applied in three main ways to protect crops. First, Bt spores have been used as natural insecticides since the 1920s. Second, purified Cry proteins can be sprayed directly onto plants, though these treatments are not durable because they are washed away by rain. Finally, genetic engineering has enabled the development of transgenic crops that produce Cry toxins themselves, providing continuous protection against insect pests (James, 2014). For example, Bt maize and Bt cotton are now widely cultivated worldwide. In addition, Bt toxins have been applied to protect stored grains such as corn, although not all storage pests are susceptible.
These examples highlight how microbial products, whether naturally occurring or engineered, serve as sustainable tools for pest management and improved crop resilience in agriculture.
Biofertilizers and Nitrogen-Fixing Microbes
Biofertilizers are living microorganisms that improve soil fertility and plant productivity by increasing the availability of essential nutrients in the rhizosphere. Unlike chemical fertilizers, which supply nutrients directly, biofertilizers stimulate natural biological processes that enrich the soil and support crop growth (Vessey, 2003).
One of the most critical roles of biofertilizers is biological nitrogen fixation, a process carried out by symbiotic bacteria that colonizes the roots of legumes. These microbes convert atmospheric nitrogen (N₂) into ammonia (NH₃) through the enzyme nitrogenase. The ammonia is then transformed into ammonium (NH₄⁺), nitrites, and nitrates, which plants can incorporate into amino acids such as glutamine and glutamate. This process is fundamental to sustainable agriculture, as it replenishes soil nitrogen levels and reduces dependence on synthetic fertilizers. It also provides the basis for crop rotation practices, where legumes enrich the soil for subsequent cereal crops (Oldroyd & Dixon, 2014).
Beyond nitrogen fixation, some beneficial bacteria function as endophytes, living inside plant vascular tissues. These endophytic microbes contribute to plant growth and resilience by enhancing drought tolerance, producing antimicrobial compounds, and priming the plant’s immune system for faster defense responses (Santoyo et al., 2016). In this way, endophytes act as an internal biofertilizer, keeping plants prepared to withstand both biotic and abiotic stresses.
Plant Growth–Promoting Rhizobacteria (PGPR)
Plant Growth–Promoting Rhizobacteria (PGPR) are a diverse group of beneficial bacteria that colonize the rhizosphere and stimulate plant growth directly or indirectly. A classic example is Pseudomonas fluorescens, which is widely studied for its antifungal activity. PGPR supports plant growth through three primary mechanisms (Glick, 2012):
- Competition for space and nutrients – PGPR effectively colonize the rhizosphere, limiting the resources available to plant pathogens. Many species produce siderophores, specialized molecules that bind tightly to iron and transport it into bacterial cells. Because iron is often a limiting nutrient in soil, siderophore production gives PGPR a competitive advantage. Importantly, many bacteria design their siderophores to be species-specific, ensuring that only the producer can access the bound iron (Neilands, 1995).
- Antibiosis – PGPR synthesize antimicrobial compounds such as hydrogen cyanide, phenazines, and other antifungal metabolites that inhibit the growth of soil-borne pathogens. This suppresses plant diseases and protects root systems.
- Induced systemic resistance (ISR) – PGPR can activate a plant’s internal defense pathways, priming the host to respond more effectively to pathogen attack. Unlike direct antibiosis, ISR does not kill pathogens immediately but strengthens the plant’s immune readiness, providing long-term protection.
Through these combined mechanisms, PGPR not only protects crops from diseases but also improve nutrient uptake, promote root development, and enhances yields in sustainable farming systems.
12.2.4 Plant growth-promoting microbes and their use in agriculture
Plant growth-promoting rhizobacteria (PGPR) can significantly facilitate the growth of many cereals and other vital crops. Different types of (PGPR) in soil suppress many plant pathogens and promote plant growth by different methods, such as direct and indirect production of different phytohormones, mineralization, and decomposition of organic matter, and improving the bioavailability of different mineral nutrients like iron and phosphorous. PGPR inhabit in the rhizosphere belongs to Achromobacter, Arthrobacter, Azotobacter, Azospirillum, Bacillus, Burkholderia, Enterobacter, Klebsiella, Microbacterium, Paenibacillus, Pantoea, Pseudomonas, Serratia, Streptomyces, etc. These PGPRs are generally used as inoculants for biostimulation, biocontrol, and biofertilization (Waqas et al., 2012).
These bacteria and other microbes improve plant growth under different environmental stress conditions. The PGPR is a naturally available source for mitigating biotic and abiotic stresses. PGPR usually improves plant growth promotion by triggering plant growth hormones and antioxidant system, producing siderophore, and enhancing the nutritional capacity of the plants. The effects of PGPR on plants include promoting growth and increased plant productivity. There are various mechanisms through which PGPR can be used for plant growth promotion, such as increased root and shoot growth by producing different phytohormones like auxins and cytokinins (Numan et al., 2018).
Indole acetic acid is an active form of auxin and an essential plant growth regulator. IAA has a crucial role in plant growth and development through its life cycle. IAA is mainly produced in actinobacteria. The strains Kitasatospora sp., Nocardia sp., and Streptomyces genus have been identified to produce IAA. The radicular system is activated by IAA root elongation, derived by apical meristem and lateral root development, increasing plant access to soil nutrients. IAA is the main auxin that promotes plant growth (Kang et al., 2014).
In addition to being a reservoir of bioactive secondary metabolites, endophytic fungi have recently been known to produce plant growth regulators. Such regulators increase plant growth and development and improve plant health by increasing tolerance against diverse environmental stresses. Plant growth regulators such as indole 3-acetic acid (IAA) and gibberellins (GAs) can stimulate rapid responses of cell elongation, cell division, and differentiation in plants(Khan et al., 2014). Some of the strains of rhizobacteria, viz., Rhizobium phaseoli, Acetobacter diazotrophicus, and Herbaspirillum seropedicae, Bacillus pumilus and B. licheniformis, B. cereus, B. macroides, and B. pumilus, Azotobacter chroococcum SE370, and Burkholderia cepacia SE4, have been known to produce GAs. Some strains of bacteria also produce IAA, which can extend growth-promoting effects during symbiosis (Verma et al., 2001). Genera such as Bacillus, Microbacterium, Methylophaga, Agromyces, and Paenibacillus have been found to produce IAA (Weyens et al., 2014).
Plant growth-promoting microbes and their use in agriculture
The growth of many cereals and other essential crops can be greatly aided by plant growth-promoting rhizobacteria (PGPR). Through a variety of processes, including the direct and indirect synthesis of various phytohormones, the mineralization and breakdown of organic matter, and the enhancement of the bioavailability of various mineral nutrients like iron and phosphorous, different types of PGPR in soil suppress a variety of plant pathogens and encourage plant growth. Achromobacter, Arthrobacter, Azotobacter, Azospirillum, Bacillus, Burkholderia, Enterobacter, Klebsiella, Microbacterium, Paenibacillus, Pantoea, Pseudomonas, Serratia, Streptomyces, and others are among the PGPR that live in the rhizosphere. These PGPRs are typically employed as biostimulation, biocontrol, and biofertilization inoculants.
Under various environmental stress circumstances, the growth of plants is enhanced by these bacteria and other microbes. One naturally occurring resource for reducing biotic and abiotic stressors is the PGPR. By stimulating the antioxidant system and plant growth hormones, generating siderophores, and improving the nutritional value of the plants, PGPR often enhances plant growth promotion. PGPR has an impact on plants by promoting growth and raising plant yield. By generating distinct phytohormones like auxins and cytokinins, PGPR can be utilized to promote plant growth in a number of ways, including greater root and shoot growth. (Numan et al., 2018).
Indole-3-acetic acid (IAA) is the most common and biologically active form of auxin, a key hormone that regulates plant growth. Throughout the plant’s life cycle, IAA shapes development by influencing processes such as cell elongation, tissue differentiation, and organ formation. Interestingly, IAA is not only synthesized by plants but also by certain soil-dwelling actinobacteria, including strains from Kitasatospora, Nocardia, and Streptomyces. In plants, IAA stimulates root system development enhancing root elongation from the apical meristem and promoting lateral root formation thereby improving the plant’s ability to absorb water and nutrients from the soil. Because of these diverse roles, IAA is often considered the principal auxin driving overall plant growth (Kang et al., 2014).
In addition to being a reservoir of bioactive secondary metabolites, endophytic fungi have recently been known to produce plant growth regulators. Such regulators increase plant growth and development and improve plant health by increasing tolerance against diverse environmental stresses. Plant growth regulators such as indole 3-acetic acid (IAA) and gibberellins (GAs) can stimulate rapid responses of cell elongation, cell division, and differentiation in plants(Khan et al., 2014). Some of the strains of rhizobacteria, viz., Rhizobium phaseoli, Acetobacter diazotrophicus, Herbaspirillum seropedicae, Bacillus pumilus, and B. licheniformis, B. cereus, B. macroides, and B. pumilus, Azotobacter chroococcum SE370, and Burkholderia cepacia SE4, have been known to produce GAs. Some strains of bacteria also produce IAA, which can extend growth-promoting effects during symbiosis. Genera such as Bacillus, Microbacterium, Methylophaga, Agromyces, and Paenibacillus have been found to produce IAA (Weyens et al., 2014).
Protection Against Viral Pathogens
Beyond insect and herbicide resistance, microbial biotechnology has also been used to develop plants with enhanced resistance to viral pathogens. Plant viruses pose a major challenge to global agriculture, often causing severe losses in staple and specialty crops. Early strategies to engineer viral resistance relied on inserting viral genes into plant genomes with the idea that the expressed protein would disrupt the virus life cycle or stimulate plant defenses, similar in concept to how vaccines work in animals (Wilson, 1993). In many cases, overproduction of viral proteins interferes with viral entry or assembly, reducing infection rates.
More recently, more precise methods have been developed, including the use of antisense constructs and inverted repeats to trigger post-transcriptional gene silencing. This mechanism targets viral mRNAs for degradation or blocks their translation, preventing the virus from replicating effectively (Prins et al., 2008). These molecular strategies have provided durable resistance against a range of plant RNA viruses.
Case Studies: Coat Protein–Mediated Viral Resistance
One of the earliest and most famous examples of viral resistance came from studies on the Tobacco Mosaic Virus (TMV), an RNA virus infecting tobacco and tomato. TMV was historically important, as it provided the first evidence of a transmissible agent smaller than bacteria, long before viruses were discovered in animals. Since then, several crops have been engineered to express viral coat proteins (CPs) to block viral replication or transmission.
Notable examples include:
- Papaya ringspot virus (PRSV): PRSV devastated papaya production in Hawaii, reducing yields by up to 95%. In 1998, transgenic papayas expressing the PRSV coat protein were introduced using a gene gun method to transform embryos. This innovation effectively revived the Hawaiian papaya industry and is often cited as a landmark success in plant biotechnology (Gonsalves, 1998).
- Plum Pox Virus (PPV): A serious threat to stone fruit crops, PPV resistance has been engineered by integrating multiple and rearranged copies of the PPV coat protein gene. While coat protein (CP) expression levels were low or undetectable, resistance was still observed in some cases, showing that even partial expression can confer protection.
- Grape Fanleaf Virus (GFLV): Transmitted by nematodes, GFLV resistance was tested by introducing the full coat protein gene into grapevine embryo suspensions using Agrobacterium tumefaciens. Although mRNA levels were low, detectable CP in some plants correlated with resistance.
- Additional trials with potato leafroll virus, cantaloupe mosaic virus, and rice stripe virus further demonstrated the broad potential of coat protein–mediated resistance.
Despite its promise, there are concerns with using full viral genes for protection. Recombination events may inadvertently generate new viral strains with enhanced virulence, expanded host ranges, or the ability to overcome existing resistance. These risks highlight the importance of careful genetic design and biosafety assessment (Hull, 2002).
12.2.1 Agrobacterium tumefaciens use in agriculture
Agrobacterium radiobacter (more commonly known as Agrobacterium tumefaciens) is the causal agent of crown gall disease (the formation of tumors) in over 140 species of eudicots (Young et al., 2001). It is a rod-shaped, Gram-negative soil bacterium (Smith & Townsend, 1907). Symptoms are caused by the insertion of a small segment of DNA (known as the T-DNA, for ‘transfer DNA,’ not to be confused with tRNA that transfers amino acids during protein synthesis) from a plasmid into the plant cell, which is incorporated at a semi-random location into the plant genome. Plant genomes can be engineered using Agrobacterium to deliver sequences hosted in T-DNA binary vectors. Agrobacterium tumefaciens is an Alphaproteobacterium of the family Rhizobiaceae, which includes the nitrogen-fixing legume symbionts.
Unlike the nitrogen-fixing symbionts, tumor-producing Agrobacterium species are pathogenic and do not benefit the plant. The variety of plants affected by agrobacterium makes it of great concern to the agriculture industry (Moore et al., 1997). Economically, A. tumefaciens is a severe pathogen of walnuts, grape vines, stone fruits, nut trees, sugar beets, horse radish, and rhubarb, and the persistent nature of the tumors or galls caused by the disease makes it particularly harmful for perennial crops (Morton & Fuqua, 2012). Agrobacterium tumefaciens grows optimally at 28 °C (82 °F). The doubling time can range from 2.5 to 4 hours depending on the media, culture format, and level of aeration. At temperatures above 30 °C, A. tumefaciens begins to experience heat shock, likely to result in errors in cell division (Weisberg et al., 2020).
12.2.2 Gene transfer through A. tumefaciens
The A. tumefaciens-mediated plant genetic transformation process requires two genetic components on the bacterial Ti-plasmid. The first essential component is the T-DNA, defined by conserved 25-base pair imperfect repeats at the ends of the T-region called border sequences. The second is the virulence (vir) region, composed of at least seven significant loci (virA, virB, virC, virD, virE, virF, and virG) encoding components of the bacterial protein machinery mediating T-DNA processing and transfer. The VirA and VirG proteins are two-component regulators that activate the expression of other vir genes on the Ti-plasmid. The VirB, VirC, VirD, VirE, and VirF are involved in the processing, transferring, and integrating the T-DNA from A. tumefaciens into a plant cell. Fig 12.3 shows the significant steps of the Agrobacterium-mediated plant transformation process.
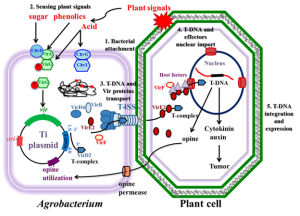
Figure 12.3. A. tumefaciens mediated transformation of Ti plasmid. This is a natural process. This figure has been adopted from “(Hwang et al., 2017)
The overall process of Ti plasmid is as follows:
- T-DNA tumor-causing genes are deleted and replaced with genes of interest (GOI) driven by plant-expressed promoters.
- Genes for DNA transfer and insertion into plant genome are retained (vir genes)
- Selectable marker genes are added to track transformed plant cells. Ex. Hygromycin (antibiotic), Bialaphos (herbicide)
- The modified Ti plasmid is constructed first in E. coli and then transformed into A. tumefaciens
- A. tumefaciens, with GOI, is co-cultured with plant leaf disks with hormone conditions favoring callus development (undifferentiated)
- Antibacterial agents are added to kill A. tumefaciens
- Hygromycin or bialaphos is added to kill non-transgenic plant cells
- Surviving cells = transgenic plant cells
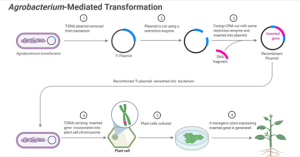
Figure 12.4. Agrobacterium-Mediated Gene Transfer (Transformation) in Plants. Created with BioRender.com
12.2.3 Genetically modified crops using microbial biotechnology approaches
- Tomato:
- Flavr Savr, a genetically modified tomato, was the first commercially grown genetically engineered food to be granted a license for human consumption. Through genetic engineering, Calgene hoped to slow down the tomato’s ripening process and thus prevent it from softening while still allowing it to retain its natural color and flavor.
- The tomato was made more resistant to rotting by adding an antisense gene that interferes with the production of the enzyme polygalacturonase (PG). The enzyme typically degrades pectin in the cell walls and results in the softening of fruit, which makes them more susceptible to being damaged by fungal infections.
- The intended effect of slowing down the softening of Flavr Savr tomatoes would allow the vine-ripe fruits to be harvested like green tomatoes without more significant damage to the tomato itself.
- The Flavr Savr disappointed researchers in that respect, as the antisense PG gene had a positive effect on shelf life, but not on the fruit’s firmness, so the tomatoes still had to be harvested like any other unmodified vine-ripe tomatoes.
b) Herbicides
- Glyphosate is a herbicide used in agriculture and non-crop situations to control many weeds.
- Chemically, the active ingredient glyphosate (N-phosphonomethyl-glycine) is a glycine derivative, the most minor amino acid found in proteins. In the glyphosate molecule, one of the amino hydrogen atoms of glycine is replaced with a phosphonomethyl group.
- Once absorbed by the plant, glyphosate binds to and blocks the activity of the enzyme enolpyruvylshikimate-3-phosphate synthase (EPSPS).
- Structural similarities to phosphoenol pyruvate enable glyphosate to bind to the substrate binding site of the EPSPS, inhibiting its activity and blocking its import into the chloroplast.
- Inhibiting the function of the shikimic acid pathway causes a deficiency in aromatic amino acids, eventually leading to the plant’s death by starvation.
c) Bacillus thuringensis crops
- Protection against insect pests in crops. BT cotton is one of the famous examples.
- Engineered with the gene for the toxic Cry (crystal; Cry1Ac!) protein from the bacterium Bacillus thuringensis
- The BT proteins are absorbed by receptors in the lining of the insect’s gut. The activated protein forms a complex that bores into the gut of an insect, causing a rupture in the gut lipid layer – eventually killing the insect.
d) other examples of crops
Golden rice, iron-enriched rice, and antioxidant-enriched tomatoes are some of the recent examples of genetically modified crops to produce high-quality food products.

Figure 12.5. Photo credits: Golden Rice Humanitarian Board © 2007; Credit: ETH Zurich / Christof Sautter; Reprinted by permission from Macmillan Publishers, Ltd: Butelli, E., et al., Nature Biotechnology 26, 1301 – 1308 copyright (2008).
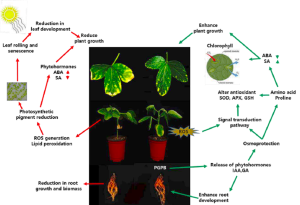
Figure 12.6. Impact of PGPR application on plant growth and development with or without stress conditions.
References:
Ahmad, P., & Prasad, M. N. V. (2011). Environmental adaptations and stress tolerance of plants in the era of climate change. Springer Science & Business Media.
Ahuja, I., de Vos, R. C., Bones, A. M., & Hall, R. D. (2010). Plant molecular stress responses face climate change. Trends in plant science, 15(12), 664-674.
Boogar, A. R., Salehi, H., & Jowkar, A. (2014). Exogenous nitric oxide alleviates oxidative damage in turfgrasses under drought stress. South African journal of botany, 92, 78-82.
Boyarshinov, A., & Asafova, E. (2011). Stress responses of wheat leaves to dehydration: participation of endogenous NO and effect of sodium nitroprusside. Russian Journal of Plant Physiology, 58(6), 1034.
Bui, L. T., Giuntoli, B., Kosmacz, M., Parlanti, S., & Licausi, F. (2015). Constitutively expressed ERF-VII transcription factors redundantly activate the core anaerobic response in Arabidopsis thaliana. Plant Science, 236, 37-43.
Chen, Q., Zhang, M., & Shen, S. (2011). Effect of salt on malondialdehyde and antioxidant enzymes in seedling roots of Jerusalem artichoke (Helianthus tuberosus L.). Acta Physiologiae Plantarum, 33(2), 273-278.
Cooke, J., & Leishman, M. R. (2016). Consistent alleviation of abiotic stress with silicon addition: a meta‐analysis. Functional Ecology, 30(8), 1340-1357.
Cui, G., Zhao, X., Liu, S., Sun, F., Zhang, C., & Xi, Y. (2017). Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiology and biochemistry, 118, 138-149.
Dawood, T., Yang, X., Visser, E. J., Te Beek, T. A., Kensche, P. R., Cristescu, S. M., Lee, S., Floková, K., Nguyen, D., & Mariani, C. (2016). A co-opted hormonal cascade activates dormant adventitious root primordia upon flooding in Solanum dulcamara. Plant Physiology, 170(4), 2351-2364.
Degenhardt, B., Gimmler, H., Hose, E., & Hartung, W. (2000). Effect of alkaline and saline substrates on ABA contents, distribution and transport in plant roots. Plant and Soil, 225(1-2), 83-94.
Eysholdt‐Derzsó, E., & Sauter, M. (2019). Hypoxia and the group VII ethylene response transcription factor HRE2 promote adventitious root elongation in Arabidopsis. Plant Biology, 21, 103-108.
Fuglsang, A. T., Guo, Y., Cuin, T. A., Qiu, Q., Song, C., Kristiansen, K. A., Bych, K., Schulz, A., Shabala, S., & Schumaker, K. S. (2007). Arabidopsis protein kinase PKS5 inhibits the plasma membrane H+-ATPase by preventing interaction with 14-3-3 protein. The Plant Cell, 19(5), 1617-1634.
Fukao, T., Xu, K., Ronald, P. C., & Bailey-Serres, J. (2006). A variable cluster of ethylene response factor–like genes regulates metabolic and developmental acclimation responses to submergence in rice. The Plant Cell, 18(8), 2021-2034.
Fukao, T., Yeung, E., & Bailey-Serres, J. (2011). The submergence tolerance regulator SUB1A mediates crosstalk between submergence and drought tolerance in rice. The Plant Cell, 23(1), 412-427.
Gasch, P., Fundinger, M., Müller, J. T., Lee, T., Bailey-Serres, J., & Mustroph, A. (2016). Redundant ERF-VII transcription factors bind to an evolutionarily conserved cis-motif to regulate hypoxia-responsive gene expression in Arabidopsis. The Plant Cell, 28(1), 160-180.
Godoy, F., Olivos-Hernández, K., Stange, C., & Handford, M. (2021). Abiotic Stress in Crop Species: Improving Tolerance by Applying Plant Metabolites. Plants, 10(2), 186.
Guo, R., Shi, L. X., Ding, X. M., Hu, Y. J., Tian, S. Y., Yan, D. F., Shao, S. A., Gao, Y. A., Liu, R., & Yang, Y. F. (2010). Effects of Saline and Alkaline Stress on Germination, Seedling Growth, and Ion Balance in Wheat [Article]. Agronomy Journal, 102(4), 1252-1260. https://doi.org/10.2134/agronj2010.0022
Hattori, Y., Nagai, K., Furukawa, S., Song, X.-J., Kawano, R., Sakakibara, H., Wu, J., Matsumoto, T., Yoshimura, A., & Kitano, H. (2009). The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature, 460(7258), 1026-1030.
Hsu, F.-C., & Shih, M.-C. (2013). Plant defense after flooding. Plant signaling & behavior, 8(11), 2699-2713.
Hurkman, W. J. (1992). Effect of salt stress on plant gene expression: a review. Plant and Soil, 146(1-2), 145-151.
Hwang, H.-H., Yu, M., & Lai, E.-M. (2017). Agrobacterium-mediated plant transformation: biology and applications. The Arabidopsis Book, 15.
Jin, H., Plaha, P., Park, J., Hong, C., Lee, I., Yang, Z., Jiang, G., Kwak, S., Liu, S., & Lee, J. (2006). Comparative EST profiles of leaf and root of Leymus chinensis, a xerophilous grass adapted to high pH sodic soil. Plant Science, 170(6), 1081-1086.
Kang, S.-M., Khan, A. L., Waqas, M., You, Y.-H., Kim, J.-H., Kim, J.-G., Hamayun, M., & Lee, I.-J. (2014). Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. Journal of Plant Interactions, 9(1), 673-682.
Khan, A. L., Waqas, M., Kang, S.-M., Al-Harrasi, A., Hussain, J., Al-Rawahi, A., Al-Khiziri, S., Ullah, I., Ali, L., & Jung, H.-Y. (2014). Bacterial endophyte Sphingomonas sp. LK11 produces gibberellins and IAA and promotes tomato plant growth. Journal of Microbiology, 52, 689-695.
Laurentius, A., & Julia, B. (2015). Flood adaptive traits and process: an overview. New Phytologist, 206(1), 57-73.
Lee, S. C., Mustroph, A., Sasidharan, R., Vashisht, D., Pedersen, O., Oosumi, T., Voesenek, L. A., & Bailey‐Serres, J. (2011). Molecular characterization of the submergence response of the Arabidopsis thaliana ecotype Columbia. New Phytologist, 190(2), 457-471.
Li, J., Xu, H.-H., Liu, W.-C., Zhang, X.-W., & Lu, Y.-T. (2015). Ethylene Inhibits Root Elongation during Alkaline Stress through AUXIN1 and Associated Changes in Auxin Accumulation. Plant physiology, 168(4), 1777-1791. https://doi.org/10.1104/pp.15.00523
Mittler, R., & Blumwald, E. (2010). Genetic engineering for modern agriculture: challenges and perspectives. Annual review of plant biology, 61, 443-462.
Moore, L. W., Chilton, W. S., & Canfield, M. L. (1997). Diversity of opines and opine-catabolizing bacteria isolated from naturally occurring crown gall tumors. Applied and Environmental Microbiology, 63(1), 201-207.
Morton, E. R., & Fuqua, C. (2012). Laboratory maintenance of Agrobacterium. Current protocols in microbiology, 24(1), 3D. 1.1-3D. 1.6.
Nanjo, Y., Jang, H.-Y., Kim, H.-S., Hiraga, S., Woo, S.-H., & Komatsu, S. (2014). Analyses of flooding tolerance of soybean varieties at emergence and varietal differences in their proteomes. Phytochemistry, 106, 25-36.
Numan, M., Bashir, S., Khan, Y., Mumtaz, R., Shinwari, Z. K., Khan, A. L., Khan, A., & Ahmed, A.-H. (2018). Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: a review. Microbiological research, 209, 21-32.
Rajhi, I., Yamauchi, T., Takahashi, H., Nishiuchi, S., Shiono, K., Watanabe, R., Mliki, A., Nagamura, Y., Tsutsumi, N., & Nishizawa, N. K. (2011). Identification of genes expressed in maize root cortical cells during lysigenous aerenchyma formation using laser microdissection and microarray analyses. New Phytologist, 190(2), 351-368.
Razi, K., & Muneer, S. (2021). Drought stress-induced physiological mechanisms, signaling pathways and molecular response of chloroplasts in common vegetable crops. Critical reviews in biotechnology, 1-40.
Sasidharan, R., Bailey‐Serres, J., Ashikari, M., Atwell, B. J., Colmer, T. D., Fagerstedt, K., Fukao, T., Geigenberger, P., Hebelstrup, K. H., & Hill, R. D. (2017). Community recommendations on terminology and procedures used in flooding and low oxygen stress research. New Phytologist, 214(4), 1403-1407.
Setter, T. L., Bhekasut, P., & Greenway, H. (2010). Desiccation of leaves after de-submergence is one cause for intolerance to complete submergence of the rice cultivar IR 42. Functional Plant Biology, 37(11), 1096-1104.
Sharma, A., Shahzad, B., Kumar, V., Kohli, S. K., Sidhu, G. P. S., Bali, A. S., Handa, N., Kapoor, D., Bhardwaj, R., & Zheng, B. (2019). Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules, 9(7), 285.
Smith, E. F., & Townsend, C. O. (1907). A plant-tumor of bacterial origin. Science, 25(643), 671-673.
Tayade, R., Nguyen, T., Oh, S. A., Hwang, Y. S., Yoon, I. S., Deshmuk, R., Jung, K.-H., & Park, S. K. (2018). Effective Strategies for Enhancing Tolerance to High-Temperature Stress in Rice during the Reproductive and Ripening Stages. Plant Breeding and Biotechnology, 6(1), 1-18.
Tunc-Ozdemir, M., Miller, G., Song, L. H., Kim, J., Sodek, A., Koussevitzky, S., Misra, A. N., Mittler, R., & Shintani, D. (2009). Thiamin Confers Enhanced Tolerance to Oxidative Stress in Arabidopsis. Plant Physiology, 151(1), 421-432. https://doi.org/10.1104/pp.109.140046
Valliyodan, B., Ye, H., Song, L., Murphy, M., Shannon, J. G., & Nguyen, H. T. (2016). Genetic diversity and genomic strategies for improving drought and waterlogging tolerance in soybeans. Journal of Experimental Botany, 68(8), 1835-1849.
van Veen, H., Akman, M., Jamar, D. C., Vreugdenhil, D., Kooiker, M., van Tienderen, P., Voesenek, L. A., Schranz, M. E., & Sasidharan, R. (2014). Group VII E thylene R esponse F actor diversification and regulation in four species from flood‐prone environments. Plant, Cell & Environment, 37(10), 2421-2432.
Verma, A., Kukreja, K., Pathak, D., Suneja, S., & Narula, N. (2001). In vitro production of plant growth regulators (PGRs) by. Indian J Microbiol, 41, 305-307.
Wang, L., Liu, J., Wang, W., & Sun, Y. (2016). Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica, 54(1), 19-27.
Waqas, M., Khan, A. L., Kamran, M., Hamayun, M., Kang, S.-M., Kim, Y.-H., & Lee, I.-J. (2012). Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules, 17(9), 10754-10773.
Weisberg, A. J., Davis, E. W., Tabima, J., Belcher, M. S., Miller, M., Kuo, C.-H., Loper, J. E., Grünwald, N. J., Putnam, M. L., & Chang, J. H. (2020). Unexpected conservation and global transmission of agrobacterial virulence plasmids. Science, 368(6495), eaba5256.
Weyens, N., Gielen, M., Beckers, B., Boulet, J., van der Lelie, D., Taghavi, S., Carleer, R., & Vangronsveld, J. (2014). Bacteria associated with yellow lupine grown on a metal‐contaminated soil: in vitro screening and in vivo evaluation for their potential to enhance Cd phytoextraction. Plant Biology, 16(5), 988-996.
Xu, W., Jia, L., Baluška, F., Ding, G., Shi, W., Ye, N., & Zhang, J. (2012). PIN2 is required for the adaptation of Arabidopsis roots to alkaline stress by modulating proton secretion. Journal of Experimental Botany, 63(17), 6105-6114. https://doi.org/10.1093/jxb/ers259
Xu, W., Jia, L., Shi, W., Baluška, F., Kronzucker, H. J., Liang, J., & Zhang, J. (2013). The tomato 14-3-3 protein TFT4 modulates H+ efflux, basipetal auxin transport, and the PKS5-J3 pathway in the root growth response to alkaline stress. Plant physiology, 163(4), 1817-1828.
Yamauchi, T., Colmer, T. D., Pedersen, O., & Nakazono, M. (2018). Regulation of root traits for internal aeration and tolerance to soil waterlogging-flooding stress. Plant Physiology, 176(2), 1118-1130.
Yang, C., Wang, P., Li, C., Shi, D., & Wang, D. (2008). Comparison of effects of salt and alkali stresses on the growth and photosynthesis of wheat. Photosynthetica, 46(1), 107-114.
Yang, Y., Qin, Y., Xie, C., Zhao, F., Zhao, J., Liu, D., Chen, S., Fuglsang, A. T., Palmgren, M. G., & Schumaker, K. S. (2010). The Arabidopsis chaperone J3 regulates the plasma membrane H+-ATPase through interaction with the PKS5 kinase. The Plant Cell, 22(4), 1313-1332.
Young, J., Kuykendall, L., Martínez-Romero, E., Kerr, A., & Sawada, H. (2001). A revision of Rhizobium Frank 1889, with an emended description of the genus, and the inclusion of all species of Agrobacterium Conn 1942 and Allorhizobium undicola de Lajudie et al. 1998 as new combinations: Rhizobium radiobacter, R. rhizogenes, R. rubi, R. undicola and R. vitis. International Journal of Systematic and Evolutionary Microbiology, 51(1), 89-103.
Zaefyzadeh, M., Quliyev, R. A., Babayeva, S. M., & Abbasov, M. A. (2009). The effect of the interaction between genotypes and drought stress on the superoxide dismutase and chlorophyll content in durum wheat landraces. Turkish Journal of biology, 33(1), 1-7.
Zhu, J.-K. (2001). Plant salt tolerance. Trends in plant science, 6(2), 66-71.
Ahmad, P., & Prasad, M. N. V. (2011). Environmental adaptations and stress tolerance of plants in the era of climate change. Springer Science & Business Media.
Boogar, A. R., Salehi, H., & Jowkar, A. (2014). Exogenous nitric oxide alleviates oxidative damage in turfgrasses under drought stress. South African journal of botany, 92, 78-82.
Dawood, T., Yang, X., Visser, E. J., Te Beek, T. A., Kensche, P. R., Cristescu, S. M., Lee, S., Floková, K., Nguyen, D., & Mariani, C. (2016). A co-opted hormonal cascade activates dormant adventitious root primordia upon flooding in Solanum dulcamara. Plant Physiology, 170(4), 2351-2364.
Fuglsang, A. T., Guo, Y., Cuin, T. A., Qiu, Q., Song, C., Kristiansen, K. A., Bych, K., Schulz, A., Shabala, S., & Schumaker, K. S. (2007). Arabidopsis protein kinase PKS5 inhibits the plasma membrane H+-ATPase by preventing interaction with 14-3-3 protein. The Plant Cell, 19(5), 1617-1634.
Fukao, T., Xu, K., Ronald, P. C., & Bailey-Serres, J. (2006). A variable cluster of ethylene response factor–like genes regulates metabolic and developmental acclimation responses to submergence in rice. The Plant Cell, 18(8), 2021-2034.
Fukao, T., Yeung, E., & Bailey-Serres, J. (2011). The submergence tolerance regulator SUB1A mediates crosstalk between submergence and drought tolerance in rice. The Plant Cell, 23(1), 412-427.
Guo, R., Shi, L. X., Ding, X. M., Hu, Y. J., Tian, S. Y., Yan, D. F., Shao, S. A., Gao, Y. A., Liu, R., & Yang, Y. F. (2010). Effects of Saline and Alkaline Stress on Germination, Seedling Growth, and Ion Balance in Wheat [Article]. Agronomy Journal, 102(4), 1252-1260. https://doi.org/10.2134/agronj2010.0022
Hwang, H.-H., Yu, M., & Lai, E.-M. (2017). Agrobacterium-mediated plant transformation: biology and applications. The Arabidopsis Book, 15.
Kang, S.-M., Khan, A. L., Waqas, M., You, Y.-H., Kim, J.-H., Kim, J.-G., Hamayun, M., & Lee, I.-J. (2014). Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. Journal of Plant Interactions, 9(1), 673-682.
Khan, A. L., Waqas, M., Kang, S.-M., Al-Harrasi, A., Hussain, J., Al-Rawahi, A., Al-Khiziri, S., Ullah, I., Ali, L., & Jung, H.-Y. (2014). Bacterial endophyte Sphingomonas sp. LK11 produces gibberellins and IAA and promotes tomato plant growth. Journal of Microbiology, 52, 689-695.
Nanjo, Y., Jang, H.-Y., Kim, H.-S., Hiraga, S., Woo, S.-H., & Komatsu, S. (2014). Analyses of flooding tolerance of soybean varieties at emergence and varietal differences in their proteomes. Phytochemistry, 106, 25-36.
Numan, M., Bashir, S., Khan, Y., Mumtaz, R., Shinwari, Z. K., Khan, A. L., Khan, A., & Ahmed, A.-H. (2018). Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: a review. Microbiological research, 209, 21-32.
Sasidharan, R., Bailey‐Serres, J., Ashikari, M., Atwell, B. J., Colmer, T. D., Fagerstedt, K., Fukao, T., Geigenberger, P., Hebelstrup, K. H., & Hill, R. D. (2017). Community recommendations on terminology and procedures used in flooding and low oxygen stress research. New Phytologist, 214(4), 1403-1407.
Sharma, A., Shahzad, B., Kumar, V., Kohli, S. K., Sidhu, G. P. S., Bali, A. S., Handa, N., Kapoor, D., Bhardwaj, R., & Zheng, B. (2019). Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules, 9(7), 285.
Tayade, R., Nguyen, T., Oh, S. A., Hwang, Y. S., Yoon, I. S., Deshmuk, R., Jung, K.-H., & Park, S. K. (2018). Effective Strategies for Enhancing Tolerance to High-Temperature Stress in Rice during the Reproductive and Ripening Stages. Plant Breeding and Biotechnology, 6(1), 1-18.
Tunc-Ozdemir, M., Miller, G., Song, L. H., Kim, J., Sodek, A., Koussevitzky, S., Misra, A. N., Mittler, R., & Shintani, D. (2009). Thiamin Confers Enhanced Tolerance to Oxidative Stress in Arabidopsis. Plant Physiology, 151(1), 421-432. https://doi.org/10.1104/pp.109.140046
Valliyodan, B., Ye, H., Song, L., Murphy, M., Shannon, J. G., & Nguyen, H. T. (2016). Genetic diversity and genomic strategies for improving drought and waterlogging tolerance in soybeans. Journal of Experimental Botany, 68(8), 1835-1849.
van Veen, H., Akman, M., Jamar, D. C., Vreugdenhil, D., Kooiker, M., van Tienderen, P., Voesenek, L. A., Schranz, M. E., & Sasidharan, R. (2014). Group VII E thylene R esponse F actor diversification and regulation in four species from flood‐prone environments. Plant, Cell & Environment, 37(10), 2421-2432.
Weisberg, A. J., Davis, E. W., Tabima, J., Belcher, M. S., Miller, M., Kuo, C.-H., Loper, J. E., Grünwald, N. J., Putnam, M. L., & Chang, J. H. (2020). Unexpected conservation and global transmission of agrobacterial virulence plasmids. Science, 368(6495), eaba5256.
Weyens, N., Gielen, M., Beckers, B., Boulet, J., van der Lelie, D., Taghavi, S., Carleer, R., & Vangronsveld, J. (2014). Bacteria associated with yellow lupine grown on a metal‐contaminated soil: in vitro screening and in vivo evaluation for their potential to enhance Cd phytoextraction. Plant Biology, 16(5), 988-996.
Xu, W., Jia, L., Baluška, F., Ding, G., Shi, W., Ye, N., & Zhang, J. (2012). PIN2 is required for the adaptation of Arabidopsis roots to alkaline stress by modulating proton secretion. Journal of Experimental Botany, 63(17), 6105-6114. https://doi.org/10.1093/jxb/ers259
Yang, Y., Qin, Y., Xie, C., Zhao, F., Zhao, J., Liu, D., Chen, S., Fuglsang, A. T., Palmgren, M. G., & Schumaker, K. S. (2010). The Arabidopsis chaperone J3 regulates the plasma membrane H+-ATPase through interaction with the PKS5 kinase. The Plant Cell, 22(4), 1313-1332.
Young, J., Kuykendall, L., Martínez-Romero, E., Kerr, A., & Sawada, H. (2001). A revision of Rhizobium Frank 1889, with an emended description of the genus, and the inclusion of all species of Agrobacterium Conn 1942 and Allorhizobium undicola de Lajudie et al. 1998 as new combinations: Rhizobium radiobacter, R. rhizogenes, R. rubi, R. undicola and R. vitis. International Journal of Systematic and Evolutionary Microbiology, 51(1), 89-103.
Bravo, A., Likitvivatanavong, S., Gill, S. S., & Soberón, M. (2011). Bacillus thuringiensis: A story of a successful bioinsecticide. Insect Biochemistry and Molecular Biology, 41(7), 423–431.
Choudhary, D. K., & Johri, B. N. (2009). Interactions of Bacillus spp. and plants – With special reference to induced systemic resistance (ISR). Microbiological Research, 164(5), 493–513.
Compant, S., Duffy, B., Nowak, J., Clément, C., & Barka, E. A. (2005). Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Applied and Environmental Microbiology, 71(9), 4951–4959.
James, C. (2014). Global status of commercialized biotech/GM crops: 2014. ISAAA Brief No. 49. Ithaca, NY: ISAAA.
Glick, B. R. (2012). Plant growth-promoting bacteria: mechanisms and applications. Scientifica, 2012, 963401.
Neilands, J. B. (1995). Siderophores: structure and function of microbial iron transport compounds. Journal of Biological Chemistry, 270(45), 26723–26726.
Oldroyd, G. E., & Dixon, R. (2014). Biotechnological solutions to the nitrogen problem. Current Opinion in Biotechnology, 26, 19–24.
Santoyo, G., Moreno-Hagelsieb, G., del Carmen Orozco-Mosqueda, M., & Glick, B. R. (2016). Plant growth-promoting bacterial endophytes. Microbiological Research, 183, 92–99.
Vessey, J. K. (2003). Plant growth promoting rhizobacteria as biofertilizers. Plant and Soil, 255(2), 571–586.
Gonsalves, D. (1998). Control of papaya ringspot virus in papaya: a case study. Annual Review of Phytopathology, 36, 415–437.
Hull, R. (2002). Matthews’ Plant Virology (4th ed.). Academic Press.
Prins, M., Laimer, M., Noris, E., Schubert, J., Wassenegger, M., & Tepfer, M. (2008). Strategies for antiviral resistance in transgenic plants. Molecular Plant Pathology, 9(1), 73–83.
Wilson, T. M. A. (1993). Strategies to protect crop plants against viruses: pathogen-derived resistance blossoms. Proceedings of the National Academy of Sciences USA, 90(8), 3134–3141.

