10 Chapter 10. Upstream Bioprocess
Hemen Hosseinzadeh and Venkatesh Balan
10.1. Introduction to Bioprocessing
10.2. Microbial and Cell Line Selection
10.3. Media Formulation and Optimization
10.4. Sterilization and Aseptic Techniques
10.6. Bioreactor Design and Operation
10.7. Environmental Control in Bioreactors
10.8. Fermentation and Cultivation Strategies
10.9. Process Monitoring and Automation
10.1. Introduction to Bioprocessing
Learning Outcomes
-
Describe the core principles of upstream bioprocessing and its role in biotechnology.
-
Identify and optimize microbial strains, media formulations, and inoculum development strategies.
-
Compare major fermentation and cultivation methods and evaluate their advantages and limitations.
-
Explain how bioreactor design, environmental control, and aseptic techniques influence yield and quality.
-
Assess the value of monitoring, automation, and upstream processing in industrial and medical applications.
Imagine a busy kitchen where chefs prepare a complex dish from scratch, by carefully combining ingredients, regulating heat, and arranging the dish with precision. This is bioprocessing: a step-by-step transformation that turns living cells into small factories to produce life-changing products such as medicines, biofuels, or food additives. As the backbone of bioproduction, bioprocessing converts genetic blueprints into practical solutions. It consists of two main phases: upstream processing, where cells are cultivated and stimulated to produce the desired product, and downstream processing, where the product is purified and formulated for final consumption. From insulin that saves lives to enzymes that convert plant waste into fuel, biotechnology is driving innovations that address some of the world’s most pressing challenges. Let’s take a look at how these processes work and why they are so important to modern biotechnology and sustainable industry.
Overview of Upstream and Downstream Processing
Downstream processing is the next phase, akin to refining a rough draft into a polished final version. Once the cells have completed production, the target product must be separated from the complex cells, media, and byproducts. If the product is intracellular, like an enzyme in E. coli, cells are disrupted using a homogenizer, and centrifugation separates the protein-rich supernatant from debris. If the product is extracellular, such as cellulases from Trichoderma reesei, filtration of the culture broth may be sufficient to isolate it. Purification is the most critical and challenging step. Think of it as sorting a laundry basket to find a single shirt. Techniques like chromatography (which separates molecules based on charge or affinity) and ultrafiltration (which filters by size) can achieve purities of 99% or higher, essential for therapeutic products like monoclonal antibodies. Purification of insulin from E. coli, for example, often requires several rounds of ion exchange chromatography to remove endotoxins and ensure patient safety. Final processing may involve drying the product into a powder or concentrating it into a stable liquid, which can then be packaged as a drug or industrial enzyme.
The strength of bioprocess engineering lies in the seamless integration of upstream and downstream processes. An optimized upstream process, e.g., P. pastoris with its methanol-inducible AOX1 promoter that secretes clean proteins, can greatly simplify downstream purification by minimizing impurities. Conversely, downstream processing challenges, such as the presence of unwanted by-products, can lead to the upstream strategy being adjusted, for example, by selecting a strain with protease activity to protect product integrity. This dynamic interplay ensures a balanced approach to maximize yield, maintain product quality, and control production costs. Figure 10.1 illustrates this interrelated workflow and shows the transition from upstream cultivation in a bioreactor to downstream purification, using methods such as chromatography to achieve the high purity standards required for industrial or pharmaceutical applications.
Importance in Biotechnology and Industrial Applications
Bioprocessing serves as the engine driving many of the most transformative breakthroughs in biotechnology and industry, converting innovative ideas into impactful real-world products. In the biomedical field, it is essential to produce life-saving therapeutics. For example, E. coli-based bioprocesses yield insulin at 3 to 5 grams per liter, making diabetes treatment more affordable and accessible. Similarly, Pichia pastoris can produce hepatitis B vaccine antigens at concentrations up to 10 grams per liter, supporting large-scale global immunization efforts. These processes go beyond manufacturing; they must comply with stringent regulatory standards set by agencies like the FDA to ensure product safety, efficacy, and consistency. Achieving this requires tightly controlled upstream processes to grow healthy, uncontaminated cells, and precise downstream steps to purify and formulate the final product.
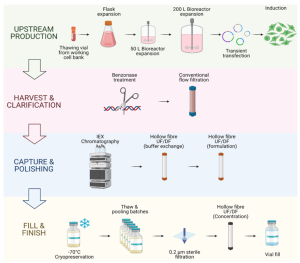
Figure 10.1: Illustration of the bioprocessing pipeline, showing upstream cell cultivation in a bioreactor and downstream product purification via chromatography. Image source: Wikimedia Commons (CC-BY-SA-4.0) (https://www.researchgate.net/figure/Example-of-an-end-to-end-upstream-and-downstream-bioprocess-for-GMP-grade-LV-vector_fig2_353688870).
In industrial applications, biological processing is a powerful tool for promoting sustainability. Aspergillus niger produces glucoamylases at a rate of 20 to 30 grams per liter, enabling the efficient conversion of starch into sugar for use in corn syrup and ethanol. Trichoderma reesei produces cellulases that break down agricultural waste into fermentable sugars for biofuel production, reducing dependence on fossil fuels. Optimized fermentation strategies can reduce production costs by 15–20%, making green technologies more economically viable. Bioprocessing also plays an important role in food and agriculture. Lactococcus lactis is used to produce enzymes for cheese production, while Bacillus thuringiensis synthesizes biopesticides that provide environmentally friendly crop protection. Biotechnology is the engine for life-saving therapies and sustainable industrial solutions. In the biomedical field, it enables the production of important drugs under strict legal requirements. , E. coli-based systems, for example, produce insulin at a rate of 3to 5 grams per liter, making the treatment of diabetes widely accessible. Similarly, Pichia pastoris produces hepatitis B vaccine antigens at up to 10 grams per liter, supporting global immunization. These systems rely on robust upstream processes to grow healthy, uncontaminated cells, and precise downstream steps to purify and formulate safe, effective products. In the industry, biotechnology ensures greater sustainability and cost efficiency. Aspergillus niger produces glucoamylases at 20–30 grams per liter for the conversion of starch to sugar in corn syrup and ethanol. Trichoderma reesei produces cellulases that break down plant biomass into fermentable sugars for biofuels. Optimized fermentations can reduce production costs by 15–20%, making green alternatives more competitive. 3 In food and agriculture, Lactococcus lactis is used in cheese production, and Bacillus thuringiensis serves as a natural biopesticide.
Bioprocessing is also expanding into frontier fields. Algae like Chlamydomonas reinhardtii and Nannochloropsis grow in photobioreactors using sunlight and CO₂ to produce omega-3 fatty acids or vaccine components, often with media costs as low as $0.50 per liter. Plants such as Nicotiana benthamiana have been used to rapidly produce antibodies in greenhouses, exemplified by ZMapp for Ebola. Economically, bioprocessing supports a biotech market now exceeding $1 trillion (2024). Yet challenges remain, downstream purification can account for 50–80% of total production costs. Innovations like using agricultural waste as feedstock and automating bioreactors with SCADA systems are helping reduce costs and environmental impact. For example, Bacillus subtilis fermentation using corn stover cut media expenses by 20%. From affordable therapeutics to renewable fuels, bioprocessing is central to a more sustainable and equitable future, and the rest of this chapter will explore how each step in the process contributes to that vision.
10.2. Microbial and Cell Line Selection
Selecting the right microbe or cell line for biomanufacturing is like picking the ideal teammate: reliable, efficient, and fit for the job. Whether it’s E. coli making insulin or CHO cells producing cancer-fighting antibodies, the choice shapes the entire bioprocess, from yield and purification ease to scalability and cost. A poor choice can lead to low productivity and high expenses, while the right one ensures efficient, cost-effective production. This section explores how strain development and cell line optimization turn microbes into high-performing producers of medicines, enzymes, biofuels, and more using cutting-edge genetic tools, smart strategies, and real-world examples.
Strain Development and Engineering
Developing a high-performance strain of microorganisms is like fine-tuning a racing car for top performance; every adjustment matters. In bioprocess engineering, microbes such as E. coli, S. cerevisiae, and B. subtilis are the workhorses, valued for their rapid growth and well-studied genetics. But to meet industrial demands, these organisms often need to be improved through strain engineering. CRISPR/Cas9, a powerful gene-editing tool, enables precise DNA modifications to increase productivity. Deleting the ackA gene in E. coli, for example, reduces acetate waste and directs resources toward 25–30% higher amylase production, a key enzyme in food processing. In Pichia pastoris, enhancement of the methanol-inducible AOX1 promoter leads to high expression of therapeutic proteins, reaching yields of 5–10 g/L. These improvements make large-scale production of enzymes, vaccines, and monoclonal antibodies such as trastuzumab more efficient, affordable, and effective for global health.
Synthetic biology offers a new level of control by designing customized genetic circuits that regulate gene expression like a dimmer switch. In Bacillus subtilis, synthetic promoters modulate the production of subtilisin, a protease used in detergents, reaching 3 g/L while reducing the costs of consumer products by 15%. These circuits enable timed gene activation and ensure that cellular resources are focused on product synthesis rather than excessive growth. In Corynebacterium glutamicum, synthetic rewiring of metabolic pathways prioritized lysine production, increasing yield for animal feed by 30%. High-throughput screening accelerates strain optimization by testing thousands of genetic variants in parallel. For example, screening 10,000 mutants of Streptomyces spectabilis identified a variant that increased antibiotic production by 20% a major advance in the fight against antibiotic resistance.
Directed evolution is another effective tool that mimics natural selection to improve microbial performance. When Synechococcus is exposed to stress conditions such as high light or salinity, strains are created that produce 1 g/L biodiesel precursors, a 25% improvement over the original strain. Similarly, in Chlamydomonas reinhardtii, a photosynthetic microalga engineered for vaccine production, evolution under high light conditions led to a 20% increase in antigen yield, supporting the development of affordable vaccines in resource-poor settings. CRISPR, synthetic biology, and directed evolution are transforming microorganisms into efficient biofactories tailored for applications ranging from agriculture to medicine, demonstrating the innovative power of modern strain engineering.
Cell Line Optimization for Productivity
The optimization of cell lines is comparable to training a top athlete; every detail, from nutrition to routine, is designed to achieve peak performance. While microorganisms such as E. coli are ideal for producing simple proteins, mammalian systems such as CHO (Chinese Hamster Ovary) cells are preferred for synthesizing complex biologics like monoclonal antibodies, which require proper folding and glycosylation for clinical efficacy. CHO cells are widely used in the pharmaceutical industry to produce drugs such as rituximab for lymphoma, but they require expensive, nutrient-rich media costing between $5 and $50 per liter. Optimization ensures that these cells remain both productive and cost-effective, a principle that also applies to microbial platforms.
With CHO cells, genetic engineering is often the first step in optimization. Increasing the expression of transcription factors such as XBP1 improves protein folding in the endoplasmic reticulum, enabling antibody production of 2–5 g/L in bioreactors. This is particularly important for high-demand biologics such as adalimumab, used to treat autoimmune diseases. Beyond genetic modifications, culture strategy is also crucial. Perfusion systems, which continuously replenish nutrients and remove waste, extend CHO productivity over several weeks and can increase yields by up to 20% compared to conventional batch cultures. These systems maintain parameters such as glucose at 2 g/L and oxygen saturation at 40% to sustain metabolic activity.
Codon optimization plays a central role in microbial systems. For example, rewriting the gene sequence of human insulin to match the tRNA preferences of Saccharomyces cerevisiae improves translation efficiency and increases yield by 40%, reaching 3 g/L. This is like giving instructions to the host in its native language, enabling faster and more accurate protein synthesis. Adaptive laboratory evolution (ALE) is another effective strategy, particularly for non-conventional hosts. In Chlamydomonas reinhardtii, exposure to high light stress (2000 µmol/m²/s) produced evolved strains capable of generating 25% more vaccine antigens, a promising advance for cost-effective vaccine production in resource-limited settings. ALE simulates natural selection but accelerates the process, producing advantageous traits within weeks rather than over evolutionary timescales. In Pichia pastoris, applying ALE under methanol stress enhances AOX1 promoter performance, increasing antibody yields to 8 g/L in high-density fermentations.
Precision tools such as machine learning further support optimization. In Bacillus subtilis, machine learning algorithms identified optimal nitrogen feeding strategies that increased subtilisin production by 15% while reducing media costs by 10%. By analyzing thousands of data points, these models also shortened development timelines by up to 30% compared to traditional trial-and-error methods. Optimization also addresses practical challenges such as protein degradation and scalability. In E. coli, silencing protease genes like pepA reduces insulin degradation, improving yield by 10% and enhancing stability during purification. In CHO cells, adding protease inhibitors to the medium helps maintain antibody integrity and ensures consistent quality for drugs like trastuzumab.
Scaling up production is another critical hurdle. Optimized S. cerevisiae strains can achieve ethanol yields of 50 g/L in 10,000-liter bioreactors, but only under tightly controlled conditions, such as pH 6.0 and aeration at 1 vvm. Automated bioreactors with real-time monitoring systems help maintain these parameters and reduce batch-to-batch variability by 15%. Emerging hosts such as Nicotiana benthamiana, a plant used for recombinant antibody production, benefit from transient expression systems. In this approach, genes are temporarily introduced via Agrobacterium tumefaciens, increasing yields by 20% in greenhouse production, as demonstrated with ZMapp, an experimental Ebola therapeutic.
Taken together, these optimization strategies, including genetic modification, ALE, computational modeling, and refinement of culture conditions, transform host cells into efficient production platforms that balance yield, cost, and scalability across applications ranging from life-saving therapeutics to sustainable industrial enzymes.
Figure 10.2. Diagram of a high-density fermentation process in a bioreactor, showcasing optimized culture conditions for S. cerevisiae producing ethanol. CC BY 4.0,
MDPI (https://www.mdpi.com/2304-8158/13/21/3407#).
10.3. Media Formulation and Optimization
Designing the ideal culture media for bioprocessing is akin to preparing a tailored smoothie for a marathon runner; every component, from sugars to vitamins, must be precisely balanced to support optimal cellular performance without causing stress or overload. Media serves as the lifeblood of biomanufacturing, supplying the essential nutrients that cells or microbes need to grow, divide, and produce valuable outputs such as proteins, biofuels, and enzymes. Poorly formulated media can deprive cells of critical nutrients or induce stress responses, reducing yields by 20% or more. In contrast, a well-optimized formulation can significantly enhance productivity while lowering costs, supporting both scalability and sustainability. This section delves into the science of nutrient optimization, customizing media for specific host organisms, and leveraging industrial or agricultural waste streams to develop eco-friendly, cost-effective feedstocks. Through real-world examples and innovative strategies, we will explore how strategic media formulation transforms cells into efficient, high-yield production platforms powering everything from therapeutic insulin to renewable bioethanol.
Nutrient Requirements
In bioprocessing, every cell is like a selective diner at a buffet, thriving only when the nutrient mix is just right. Whether it’s E. coli producing insulin or CHO cells manufacturing antibodies, the culture media must deliver carbon, nitrogen, phosphorus, vitamins, and trace elements in precisely balanced amounts. An imbalanced formulation can slow growth, trigger stress responses, or even lead to cell death. Get it right, and you unlock yields that make industrial-scale production viable. Carbon sources such as glucose or glycerol serve as the primary energy supply. For example, E. coli grows rapidly with 10–20 g/L of glucose, doubling its biomass every 20 minutes and producing insulin at up to 3 g/L in bioreactors (Figure 10.3). However, excess glucose can trigger overflow metabolism, resulting in acetate accumulation, pH drops, and yield losses of up to 15%. Nitrogen, typically provided as ammonium chloride or urea, is essential for protein synthesis. B. subtilis, for instance, requires 1–2 g/L of ammonium to produce subtilisin (a detergent enzyme) at yields of 2 g/L. Phosphorus, usually supplied as phosphate salts, supports DNA replication and ATP generation. Even modest deficiencies can halt cell growth. For example, S. cerevisiae needs about 0.5 g/L of phosphate to maintain ethanol production at 50 g/L.
Vitamins and trace elements play a critical yet subtle role in bioprocessing, small in quantity but essential for optimal cell function. Compounds like biotin and vitamin B12 are vital cofactors in Pichia pastoris, supporting the AOX1 pathway for efficient antibody production at concentrations of 5 to 10 grams per liter. Similarly, trace metals such as magnesium, zinc, and iron are indispensable enzyme cofactors. For instance, supplementing C. glutamicum with 10 milligrams per liter of zinc can enhance lysine output by up to 25%, benefiting its use in animal feed. Mammalian cells, such as CHO lines, have significantly more complex nutritional demands. Their culture media must include a tailored mix of amino acids, growth factors, and hormones like insulin-like growth factor (IGF-1) to support the production of therapeutics such as rituximab, typically reaching yields around 2 grams per liter. These customized media can cost between $5 and $50 per liter, a stark contrast to the $0.10–$1 per liter required for microbial systems like E. coli. Nutrient balancing is both an art and a science. Stoichiometric modeling and metabolic pathway analysis help fine-tune formulations, delivering just the right amount of nutrients to maximize productivity without waste. For example, excessive nitrogen in Aspergillus niger cultures can redirect metabolism away from glucoamylase synthesis, leading to a 10% drop in enzyme yield. Such precision in media design is crucial for maintaining healthy, productive cells and achieving cost-effective, high-yield bioprocessing.
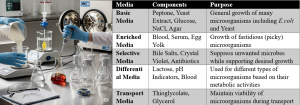
Figure 10.3. Media preparation steps and examples of different media, ingredients, and purposes. Image created with Google Gemini. Released under CC BY 4.0
Microbial and Cell-Specific Media Needs
Designing culture media is like tailoring a custom-made suit; each organism has specific nutritional and environmental needs that must be met to optimize growth, productivity, and cost-effectiveness. A one-size-fits-all approach simply won’t work across diverse microbial and cell systems. E. coli thrives in simple media such as Luria-Bertani broth supplemented with glucose and yeast extract, achieving insulin yields of 3 g/L when pH is maintained at 7.0 and oxygen saturation above 30%. Pichia pastoris relies on methanol (1–4% v/v) to induce the AOX1 promoter, producing up to 8 g/L of antibodies, though tight methanol control is critical to avoid toxicity and a 20% yield drop. S. cerevisiae prefers slightly acidic glucose-rich media (pH 6.0, 50 g/L glucose), supporting ethanol production of 50 g/L in fed-batch cultures. Design of Experiments (DoE) further refines formulation; in Corynebacterium glutamicum, adjusting glucose and ammonium improved lysine output by 25%.
Fungi like A. niger prefer acidic (pH 5.0) starch-based media such as cornmeal, producing glucoamylase at 20 g/L. Solid-state fermentation using wheat bran lowers water use and media costs by 15%. For photosynthetic microalgae like C. reinhardtii, TAP medium (~$0.50/L) supports production of omega-3 fatty acids and vaccine antigens at 1 g/L, with light and CO₂ supply being essential for yield maintenance. Mammalian CHO cells require serum-free media with tightly controlled pH (7.0–7.4), osmolarity (280–320 mOsm), and growth factors like IGF-1 to reach antibody yields of 2 g/L. Metabolomics and systems biology tools can further optimize these formulations; for example, tuning nutrient uptake in B. subtilis increased subtilisin production by 15%.
Integrating Agricultural Waste as Feedstock
To enhance sustainability and cut costs, bioprocesses increasingly turn to industrial and agricultural waste as alternative feedstocks, transforming byproducts into high-value products. This approach aligns with circular economy principles but presents challenges like variability and the need for contaminant removal. A. niger can utilize agricultural residues such as corn stover and sugarcane bagasse to produce glucoamylase at 20 g/L for bioethanol applications. Corn stover costs just $0.05/kg, significantly cheaper than glucose ($0.50/kg), though enzymatic hydrolysis to break down lignin adds roughly 10% to overall costs. Lactococcus lactis uses whey, a cheese-making byproduct, to produce nisin at 1 g/L, reducing raw material costs by 30%. Industrial waste glycerol from biodiesel production serves as a low-cost carbon source for E. coli, yielding 2 g/L of recombinant proteins like insulin for just $0.10/L, though purification is needed to remove impurities.
Other examples include T. reesei, which uses brewery spent grain to produce cellulases at 15 g/L, lowering costs by 25%. Though seasonal variations in feedstock can reduce yields by 5–10%, preprocessing strategies like steam explosion help standardize input material and boost Bacillus subtilis subtilisin yields by 10%. Microalgae like C. reinhardtii can grow on wastewater containing nitrogen from sewage, cutting media costs to $0.30/L while producing omega-3 fatty acids, provided heavy metals are removed to prevent toxicity. By combining organism-specific media design with smart use of waste-derived feedstocks, bioprocessing can maximize productivity, lower costs, and minimize environmental impact. This integrated strategy supports both economic and ecological goals, transforming waste into wealth and advancing sustainable biomanufacturing for products ranging from biofuels to life-saving therapeutics.
10.4. Sterilization and Aseptic Techniques
Sterilization and aseptic techniques are essential safeguards in bioprocessing, ensuring that only the intended microbes or cells, like E. coli or CHO cells, thrive in the bioreactor. Much like preparing a sterile operating room for surgery, even a single contaminant can jeopardize the entire process, ruining batches of life-saving antibodies or disrupting biofuel production. Contamination can reduce yields by up to 90% and compromise product safety, especially in pharmaceuticals and food-grade enzyme production. This section explores the critical steps for sterilizing equipment, media, and air, and the protocols that maintain contamination-free environments. Through practical examples, we highlight how these techniques underpin successful biomanufacturing from antibiotic synthesis to the development of sustainable bioplastics.
Sterilization of Equipment, Media, and Air
Sterilization is the foundation of a clean bioprocess, like wiping down a kitchen counter before preparing a gourmet meal. It eliminates all microbial life, including bacteria, fungi, spores, and viruses, to ensure only the desired organism thrives in the bioreactor. This step targets three critical areas: equipment, media, and air, each requiring tailored methods to achieve sterility without compromising performance or nutrient integrity. Bioreactors, the centerpiece of bioprocessing, range from 2-liter lab vessels to 20,000-liter industrial tanks, all of which must be contaminant-free before use. Steam sterilization, commonly via autoclaving, is the gold standard, using high-pressure steam at 121°C and 15 psi for 20–30 minutes to destroy even the most resilient spores, such as Geobacillus stearothermophilus. For instance, in Kluyveromyces marxianus fermentations for bioethanol, autoclaving prevents wild yeast contamination, supporting yields of up to 55 g/L. At industrial scales, steam-in-place (SIP) systems circulate steam throughout tanks, pipes, and valves to sterilize every surface. A 15,000-liter SIP cycle for Pseudomonas fluorescens enzyme production typically takes 45–60 minutes, averting contamination that could result in $60,000 losses per batch.
Heat-sensitive components like pH probes or flexible tubing are sterilized with vaporized hydrogen peroxide (VHP), which achieves a 99.9999% microbial kill rate without damaging sensitive materials. This is essential for CHO cell cultures producing monoclonal antibodies such as bevacizumab. The media, the nutrient broth fueling cell growth, is particularly prone to contamination. Liquid media like yeast nitrogen base (YNB) for Yarrowia lipolytica lipid production are autoclaved at 121°C for 15 minutes to ensure sterility and enable omega-3 yields of 2 g/L. Heat-labile nutrients, including vitamins for HEK293 cell cultures used in viral vector production, are filter-sterilized through 0.22-micron membranes to preserve their bioactivity. Similarly, Pichia pastoris fermentations rely on filter-sterilized methanol to drive AOX1-regulated protein expression at yields of 6 g/L. In solid-state fermentation, substrates like corn bran for Rhizopus oryzae lactic acid production are steam-treated at 100°C in rotating drum sterilizers, maintaining structural integrity and supporting yields around 10 g/L. Without sterilization, microbial competition can cut yields by up to 30%.
Air entering bioreactors must be sterile; one airborne spore can multiply into billions within hours, threatening the entire process. High-efficiency particulate air (HEPA) filters, capable of capturing 99.9% of particles down to 0.3 microns, are standard in processes like Lactobacillus plantarum fermentations, where probiotic concentrations reach 10¹⁰ CFU/mL. In Spirulina platensis photobioreactors, incoming air is first passed through UV sterilizers before bubbling through the culture, preventing algal contaminants and supporting protein yields of 1 g/L for nutritional supplements. For Streptomyces griseus streptomycin production, multi-stage filtration systems combining coarse pre-filters with HEPA filters maintain sterility at airflow rates of 1,500 liters per minute, even in 12,000-liter bioreactors. These air sterilization strategies are essential, as airborne contamination can reduce yields by up to 70% in high-density aerobic systems.
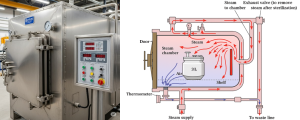
Figure 10.4. Front and cross-sectional views of a steam autoclave sterilizing a 3-liter bioreactor vessel, illustrating steam circulation, air displacement, and key components like the exhaust valve and thermometer. Image created with Google Gemini. Released under CC BY 4.0
Contamination Control Strategies
Maintaining sterility in bioprocessing is like defending a fortress; once secured, it requires constant vigilance to keep intruders out. Contamination control strategies are essential to prevent microbes, spores, or bacteriophages from breaching the system, where even a single lapse can be catastrophic. For example, a bacteriophage infection in a Lactococcus lactis culture can wipe out up to 95% of cells within hours, halting nisin production used in food preservation. Effective control relies on a combination of engineering safeguards, biological barriers, and strict operational protocols to preserve process integrity and ensure consistent, high-quality output.
Aseptic techniques serve as the frontline defense in bioprocessin,g like working within a sterile bubble to keep contaminants out. During Y. lipolytica inoculum transfers, laminar flow hoods with HEPA-filtered air create a sterile zone that reduces contamination risk by up to 98%. Operators wear sterile gloves and gowns, and use ethanol-sanitized tools, all in strict compliance with good manufacturing practices (GMP) to prevent microbial intrusion. In Saccharomyces cerevisiae bioethanol production, closed piping systems with sterile connectors allow media additions without exposure, supporting stable yields of 60 g/L. Similarly, in Spirulina platensis cultures, automated sampling ports minimize manual handling and cut contamination risk by 85%. These techniques demand rigorous training and attention to detail; human error alone can reduce yields by as much as 20%.
Bioreactor design plays a critical role in contamination prevention. Sealed systems made of polished stainless steel with sterile gaskets minimize leaks, a key factor in Pseudomonas fluorescens enzyme production, where fungal contamination can halve yields. For K. marxianus fermentations, double mechanical seals on agitator shafts flushed with sterile condensate block potential entry points and help maintain bioethanol production stability. Real-time monitoring systems, such as SCADA (supervisory control and data acquisition), provide early alerts to pressure drops or microbial growth, allowing operators to respond within minutes. In HEK293 cell cultures, ATP-based bioluminescence assays are used to detect contamination every eight hours, helping prevent batch losses in viral vector production. These monitoring tools are critical, capable of saving up to 75% of batches in high-value applications like gene therapy, where a single batch can be worth over $200,000.
Biological defenses provide an additional layer of protection in bioprocessing. For example, Lactococcus lactis engineered with plasmid-encoded phage resistance systems such as restriction-modification genes can reduce infection-related losses by up to 92%, safeguarding nisin production at yields of 1 g/L. In Rhizopus oryzae fermentations, the addition of natural antifungal compounds like natamycin suppresses mold growth, maintaining stable lactic acid output for bioplastic applications. Chemical controls also play a role: low-dose sanitizers used in Spirulina platensis cultures effectively prevent bacterial contamination without harming the algae, supporting consistent protein yields. Antifoam agents, such as polyalkylene glycols, are commonly used in Saccharomyces cerevisiae bioreactors to manage foam formation and an often-overlooked contamination pathway. Unchecked foam can breach vessel seals and reduce ethanol yields by as much as 15%.
Environmental controls extend contamination prevention beyond the bioreactor. Cleanroom facilities rated ISO Class 5 dramatically reduce airborne microbial loads by up to 97% during CHO cell-based antibody production, enabling consistent yields of 3 g/L for biologics like bevacizumab. Positive pressure within bioreactor systems helps prevent unfiltered air from entering, which is especially critical for large-scale Streptomyces griseus antibiotic fermentations in 12,000-liter tanks. Routine environmental monitoring, including surface swabbing and air quality testing, ensures cleanroom integrity and early detection of potential breaches. Failure to maintain these standards can result in contamination events costing over $100,000 in lost production.
10.5. Inoculum Development
Inoculum development is like raising a small, elite team of athletes before sending them into a stadium-sized competition. These carefully prepared starter cultures serve as the foundation of any bioprocess, providing a strong, active population of cells ready to drive the production of enzymes, therapeutics, biofuels, and more. A poorly developed inoculum can significantly hinder performance, leading to production delays, yield losses of up to 20%, and increased variability that complicates scale-up. In contrast, a well-optimized inoculum ensures consistency, efficiency, and smoother transitions to larger bioreactors. This section delves into the precise steps involved in preparing and scaling up inoculum for industrial applications, highlighting how this critical phase sets the stage for biomanufacturing success. Through real-world examples and practical insights, we’ll explore how robust inoculum strategies fuel the efficient production of everything from insulin to sustainable bioplastics, ensuring that cells are primed for peak performance at every stage of the process.
Preparation of Starter Cultures
Preparing starter cultures is like nurturing seedlings in a greenhouse before transplanting them into a larger field. The aim is to cultivate a healthy, uniform population of cells that will thrive in the bioreactor and efficiently produce the target product. This process begins on a small scale, often with a single colony or a vial of frozen stock, and requires precise control of conditions to ensure cell vigor and sterility. For E. coli producing recombinant proteins such as human growth hormone, the process typically starts by picking a single colony from an agar plate and inoculating it into 5–10 mL of Luria-Bertani (LB) broth. The culture is incubated at 37°C while shaking at 250 rpm (Figure 10.5), reaching a cell density of approximately 10⁹ cells/mL within 6 to 8 hours. The media is optimized with 10 g/L glucose and pH 7.0 to support rapid, stress-free growth, enabling protein yields of 2.5 g/L when driven by the T7 promoter. Monitoring the microbial growth phases, lag, exponential, and stationary, is essential to harvesting cells at their most productive stage. In B. licheniformis fermentations for protease production, cultures are harvested during the exponential phase at an optical density of 0.6 (600 nm), maximizing enzyme yields of 2 g/L. Automated shaking incubators equipped with real-time OD sensors enhance consistency, reducing batch variability by 10% compared to manual monitoring.

Figure 10.5. Illustration of microbial inoculum preparation and seed train progression. The top left panel shows E. coli inoculum preparation in LB medium. The top middle panel depicts CHO cell expansion on culture plates. The top right panel illustrates solid-state fermentation used for preparing bacterial or fungal inoculum. The bottom panel shows a schematic of the seed train process from initial cell culture to the main production bioreactor. Industrial-scale systems, including a stirred-tank fermenter (liquid fermentation), a horizontal fermenter (solid-state fermentation), and a tubular photobioreactor, are depicted on the right. Image created with Google Gemini. Released under CC BY 4.0
For Pichia pastoris, commonly used in antibody production, starter cultures are grown in yeast extract, peptone, and dextrose (YPD) medium supplemented with 1% methanol to induce the AOX1 promoter. Cultures typically reach 10⁸ cells/mL within 12 hours, ensuring cells are metabolically active and primed for high-yield fermentation, supporting antibody production up to 6 g/L in downstream bioreactors. To maintain long-term consistency, starter cultures are cryopreserved at −80°C in glycerol stocks. For Yarrowia lipolytica, used in lipid production, this practice preserves genetic stability and ensures reproducible yields of 1.5 g/L across multiple fermentation runs.
Eukaryotic cells, such as CHO cells used to produce monoclonal antibodies like pembrolizumab, require complex and nutrient-rich media. These formulations typically include essential amino acids and either fetal bovine serum or serum-free alternatives, costing between $10 and $50 per liter. CHO cultures are initiated at a 50 mL scale, incubated at 37°C with 5% CO₂, and reach densities of 10⁶ cells/mL within 48 hours. Maintaining precise control of pH (7.2) and dissolved oxygen (40%) minimizes cellular stress and primes the cells for high-yield antibody production, reaching up to 3 g/L. For Spirulina platensis, a cyanobacterium cultivated for food-grade proteins, starter cultures are grown in Zarrouk’s medium under high-intensity light (1,500 µmol/m²/s), achieving a biomass concentration of 0.5 g/L in 5 days. Light intensity and CO₂ availability are critical parameters; deviations can lower protein yields by up to 15%.
Scale-Up Strategy for Inoculum
Scaling up inoculum is like growing a small startup into a global corporation, which requires strategic planning to maintain quality, consistency, and performance at every level. The process typically progresses from shaking flasks to seed bioreactors, and ultimately to large-scale production tanks, with careful monitoring to ensure cells remain healthy and productive throughout. Poorly managed scale-up can result in extended lag phases, nutrient imbalances, or contamination, potentially delaying production by up to 24 hours or reducing yields by as much as 25%. A common strategy involves a stepwise 1:10 scale-up ratio, which helps minimize dilution shock between stages. For example, in E. coli systems producing human growth hormone, a 10 mL starter culture is transferred to a 100 mL flask, then to a 1-liter seed bioreactor, and finally into a 10,000-liter production tank (Figure 10.5). Maintaining a cell density of 10⁸ cells/mL at each stage supports consistent growth and enables protein yields of approximately 2.5 g/L. In Pichia pastoris, scale-up involves controlled methanol feeding (1% v/v) to sustain AOX1 promoter activity, reaching antibody yields of up to 6 g/L in 5,000-liter bioreactors. The use of automated seed bioreactors maintaining pH at 6.0 and dissolved oxygen at 30% can significantly improve efficiency, reducing lag phases by up to 12 hours compared to manual processes.
On the other hand, scaling up more complex systems such as CHO cells, phototrophic organisms, and solid-state fermentations requires specialized strategies tailored to the organism and product (Figure 10.6). For CHO cells, scale-up typically begins in 500 mL spinner flasks, progresses to 10-liter wave bioreactors, and culminates in 10,000-liter stirred-tank reactors. Maintaining shear stress below 0.5 Pa is crucial to prevent cell damage, supporting antibody yields of up to 3 g/L. Perfusion systems, which continuously refresh the culture medium, enhance cell viability and increase productivity by approximately 20% compared to batch systems. Solid-state fermentation presents unique challenges. For Rhizopus oryzae used in lactic acid production, the process starts with inoculating solid substrates like wheat bran in 1-kg trays and scales up to 100-kg rotating drum bioreactors. Maintaining 70% moisture is critical to achieving yields around 10 g/L. In the case of Bacillus licheniformis for protease production, solid inoculum is first cultivated in small fermenters before scaling to 5,000-liter systems, ensuring consistent yields of 2 g/L. For Spirulina platensis, a phototrophic microalga, scale-up takes place in photobioreactors starting with 1-liter flat-panel systems and moving to 1,000-liter tubular reactors. Light intensity is gradually increased from 1,500 to 3,000 µmol/m²/s to mimic production conditions, resulting in a 15% improvement in protein yield, reaching 1 g/L.
Advanced tools such as computational fluid dynamics (CFD) are used to optimize mixing and aeration in seed bioreactors, minimizing oxygen gradients that can otherwise reduce lipid yields in Yarrowia lipolytica by up to 10%. Contamination control is equally critical, as sterile transfer systems help prevent losses, which have been reported in up to 30% of poorly managed S. cerevisiae ethanol batches. Monitoring cell physiology is essential throughout scale-up. For E. coli, flow cytometry ensures that over 95% of cells remain viable during transfer to production tanks. For Pichia pastoris, real-time biomass sensors help maintain optimal cell density (around 100 g/L wet weight), significantly boosting antibody production. These strategies, including gradual volume increases, environmental control, and real-time monitoring, enable seamless inoculum scale-up, ensuring consistent and high-yield production of valuable bioproducts such as bioplastics, therapeutics, and biofuels.
10.6. Bioreactor Design and Operation
Bioreactors are the core of modern biomanufacturing, high-tech vessels where cells and microbes transform raw materials into valuable products ranging from life-saving therapeutics to sustainable biofuels. These controlled environments enable optimal cell growth, metabolism, and product synthesis, supporting the production of enzymes, antibodies, vaccines, and more. Designing and operating bioreactors requires a careful balance of engineering precision and biological understanding to maintain ideal conditions while ensuring cost-efficiency and scalability. Poorly designed systems can severely impact performance. Insufficient oxygen or excessive shear stress may reduce yields by over 30%. This section examines the major types of bioreactors, including stirred-tank, airlift, packed-bed, and photobioreactors, and the key principles behind their design and scale-up. Through practical examples and real-world applications, we’ll explore how these systems drive efficient and sustainable bioprocessing, powering the production of everything from insulin to bioplastics.
Types of Bioreactors
Bioreactors come in a variety of designs, each optimized for specific organisms and products, much like selecting the right tool for a particular task. Whether it’s a robust stirred-tank reactor for high-density bacterial fermentation or a gentle airlift system suited for delicate mammalian cells, the choice of bioreactor plays a pivotal role in determining the efficiency, scalability, and success of the bioprocess.
(i) Airlift Bioreactors: Airlift bioreactors operate like a bubbling fountain, using gas sparging to circulate liquid without mechanical agitation. This low-shear environment is ideal for fragile cells such as CHO cells producing monoclonal antibodies like pembrolizumab, reaching titers of 3 g/L in 5,000-liter systems (Figure 10.6). They are also used for cultivating Lactobacillus plantarum probiotics, maintaining 10¹⁰ CFU/mL while consuming 30% less energy than stirred-tank reactors. Although their simple design reduces maintenance, mixing efficiency can be limited in high-viscosity cultures.
(ii) Stirred Tank Bioreactors: Stirred tank bioreactors are the workhorses of bioprocessing, like versatile systems akin to industrial blenders, mixing cells, nutrients, and gases with rotating impellers (100–600 rpm). Their robust design makes them ideal for hardy organisms like E. coli, which can produce recombinant human growth hormone at yields of 2.5 g/L in 10,000-liter vessels, supported by high oxygen transfer rates (up to 150 mmol/L/h). These bioreactors also accommodate diverse products, from B. licheniformis proteases (2 g/L) to Saccharomyces cerevisiae bioethanol (60 g/L). As illustrated in Figure 10.5, the combination of impellers and spargers ensures uniform distribution of nutrients and oxygen, which is critical for maximizing productivity. Their flexibility allows precise control of environmental conditions, but the high shear forces generated can damage shear-sensitive eukaryotic cells, necessitating careful optimization for such systems. Systems akin to industrial blenders, mixing cells, nutrients, and gases with rotating impellers (100–600 rpm). Their robust design makes them ideal for hardy organisms like E. coli, which can produce recombinant human growth hormone at yields of 2.5 g/L in 10,000-liter vessels, supported by high oxygen transfer rates (up to 150 mmol/L/h). These bioreactors also accommodate diverse products, from Bacillus licheniformis proteases (2 g/L) to Saccharomyces cerevisiae bioethanol (60 g/L). As illustrated in Figure 10.5, the combination of impellers and spargers ensures uniform distribution of nutrients and oxygen, which is critical for maximizing productivity. Their flexibility allows precise control of environmental conditions, but the high shear forces generated can damage shear-sensitive eukaryotic cells, necessitating careful optimization for such systems.
(iii) Packed Bed Bioreactors: Packed bed bioreactors immobilize cells on solid supports, much like a garden trellis that anchors climbing plants. This setup is ideal for continuous production, as the cells stay fixed while fresh medium flows through the bed. For instance, Rhizopus oryzae produces lactic acid for bioplastic synthesis at concentrations of 10 grams per liter using polyurethane foam as the support matrix, enabling sustained week-long production cycles. These systems are particularly well-suited for fungi and biofilm-forming organisms like Pseudomonas fluorescens used in enzyme production. However, excessive biomass accumulation can clog the bed and reduce flow efficiency by up to 15%, necessitating periodic cleaning to maintain performance.
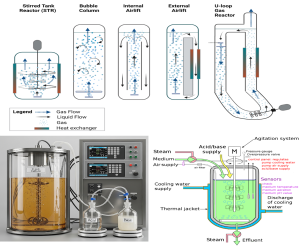
Figure 10.6. Various liquid-phase bioprocessing systems are illustrated, including mechanically agitated vessels, gas-lift configurations, and loop-based designs (developed using BioRender.com). A stirred-tank fermenter growing E. coli is shown alongside its working principle, highlighting impeller-driven mixing and oxygen sparging to promote optimal cell growth and bioproduct formation. Image licensed under CC by, Shutterstock (ID: 2315853545). (https://commons.wikimedia.org/wiki/File:Real_life_bioreactor.png). https://gfi.org/science/the-science-of-fermentation/deep-dive-fermentation-upstream-bioprocess-design/ , and Google Gemini. Released under CC BY 4.0
(iv) Photobioreactors: Photobioreactors harness light to cultivate photosynthetic organisms, functioning like solar-powered greenhouses. Spirulina platensis thrives in tubular photobioreactors under high light intensity (2000 µmol/m²/s), producing protein at concentrations of 1 gram per liter for nutritional supplements. Flat-panel photobioreactors enhance light distribution and are used for Nannochloropsis salina, yielding 1.5 grams per liter of lipids for biofuel production, while reducing energy consumption by 25% compared to stirred-tank systems. Although open pond systems offer lower capital costs, they are more susceptible to contamination. For high-value products, such as vaccine antigens from Chlamydomonas reinhardtii, closed photobioreactors are preferred due to their sterility and process control. Ultimately, the choice of bioreactor depends on the target organism, desired product, and trade-offs between efficiency, cost, and scalability.
Design Considerations and Scale-Up
Designing and scaling up bioreactors is akin to constructing a skyscraper from a blueprint; each level must support the next without compromising integrity. The challenge lies in preserving optimal conditions for oxygen transfer, mixing, temperature control, and shear stress as the system expands from lab scale (1–10 liters) to industrial volumes (1,000–20,000 liters), all while maintaining consistent productivity and product quality.
(i) Oxygen Transfer and Mixing: Oxygen transfer is a major bottleneck in high-density cell cultivation, especially for organisms like E. coli, which demand oxygen uptake rates of 100–200 mmol/L/h. In small-scale bioreactors, sparging air at 1 vvm (volume of air per volume of liquid per minute) typically suffices. However, scaling up to 10,000-liter tanks requires either larger impellers or increased airflow rates, often up to 2 vvm, leading to a 20% rise in energy consumption. For Pichia pastoris expressing antibodies under the AOX1 promoter, oxygen delivery must support yields of up to 6 g/L, necessitating advanced sparger designs that reduce bubble size and enhance mass transfer efficiency. Computational fluid dynamics (CFD) simulations have proven effective in optimizing impeller geometry, improving oxygen transfer rates by up to 15% in Saccharomyces cerevisiae fermentations, enabling ethanol titers of 60 g/L at scale.
(ii) Temperature and Heat Management: Effective temperature control is essential to avoid thermal stress and preserve product yield. Metabolic activity from organisms like Bacillus licheniformis can increase bioreactor temperatures by up to 5°C, which, if unmanaged, can lead to protein misfolding or cell death. In E. coli fermentations, jacketed vessels and internal heat exchangers are used to maintain the optimal 37°C, supporting protein yields of around 2.5 g/L. However, in large-scale systems (e.g., 10,000-liter Rhizopus oryzae fermentations), heat removal becomes increasingly difficult. External cooling loops capable of dissipating up to 50 kW of metabolic heat are often required, adding approximately 10% to operational costs. Failure to manage heat effectively can reduce yields by up to 20% due to thermal denaturation or stress-induced metabolic shifts
(iii) Shear Stress and Cell Viability: Mechanical shear from impellers or gas bubbles can significantly impair the viability of shear-sensitive cells. For example, CHO cells used in monoclonal antibody production exhibit optimal growth under shear conditions below 0.5 Pa; exceeding this threshold can reduce product titers by 10% or more. To mitigate this, airlift bioreactors or bubble-free aeration systems such as silicone membrane diffusers are increasingly used, especially for fragile lines like HEK293 cells producing viral vectors, where maintaining concentrations of 10⁸ viral particles/mL is critical. Similarly, in Spirulina platensis cultures, gentle agitation in tubular photobioreactors helps preserve filament integrity and maximize protein production. As scale increases, so does turbulence, making shear control a key engineering challenge during process intensification.
Scale-Up Challenges
Scaling up bioprocesses requires maintaining geometric similarity to preserve fluid dynamics and mixing behavior. For P. pastoris, increasing bioreactor volume from 10 to 5,000 liters necessitates raising impeller speeds from 200 to 400 rpm, but this can introduce excessive shear, potentially reducing antibody yields. Computational fluid dynamics (CFD) simulations help maintain uniform oxygen gradients, improving production efficiency by up to 10%. Foam formation presents another challenge; for example, S. cerevisiae ethanol fermentations often generate persistent foam that clogs and reduces output by 15%. Silicone-based antifoams effectively suppress foam, supporting consistent yields of 60 g/L. Modular bioreactor systems with standardized components streamline scale-up and reduce capital costs by 20% in N. salina lipid production. Careful control of oxygen transfer, temperature, shear stress, and foam ensures reliable performance across scales, enabling efficient and reproducible industrial biomanufacturing.
10.7. Environmental Control in Bioreactors
Operating a bioreactor is like conducting a symphony; every element must be in perfect harmony. Temperature, pH, dissolved oxygen, agitation, and aeration each play a vital role in orchestrating optimal cell performance. These environmental parameters form the backbone of bioprocessing, ensuring organisms like E. coli or CHO cells thrive and deliver high yields of proteins, enzymes, biofuels, or biopolymers. Even small deviations a 1°C temperature shift or slight pH imbalance can stress cells and reduce yields by 20% or more. Bioreactors function as precisely curated environments, tailored to the specific needs of the cells and the target product. Maintaining control over these parameters requires more than routine monitoring; it demands advanced sensors and automated process control systems capable of real-time adjustments. This section explores how the tight regulation of environmental conditions supported by modern technologies ensures consistent, high-quality production across applications, from monoclonal antibodies to sustainable bioplastics. Through practical examples and fresh insights, we’ll see how keeping cells “happy” translates directly into industrial success.
(i) Temperature: Temperature control is like setting the thermostat in a finely tuned lab home essential for cellular comfort and productivity. E. coli, commonly used for producing recombinant enzymes such as β-galactosidase, performs optimally at 37°C, yielding up to 2 grams per liter. Even a small 2°C shift can disrupt metabolic pathways, slashing productivity by 15%. For Pichia pastoris expressing insulin under the AOX1 promoter, 30°C supports proper protein folding and maintains yields around 5 grams per liter. In large-scale fermentations, such as a 10,000-liter Bacillus subtilis bioreactor producing α-amylase (1.5 g/L), metabolic heat can raise internal temperatures by up to 4°C. To counteract this, cooling jackets and heat exchangers are employed to maintain thermal balance. Photosynthetic organisms like Chlorella vulgaris, cultivated for biofuel lipids, require lower temperatures, typically around 25°C, in photobioreactors. Exceeding this optimal range can suppress photosynthesis by 20%, significantly affecting lipid productivity (1.2 g/L). In all systems, precise thermal regulation is vital to protect product quality and ensure process efficiency.
(ii) pH: pH control is like fine-tuning the acidity of a recipe to achieve optimal results. S. cerevisiae, used for bioethanol production, performs best at pH 5.5–6.0, yielding up to 55 g/L, while a 0.5-unit shift can reduce output by 10% due to enzyme inhibition. Lactobacillus casei, producing lactic acid for food applications, thrives at pH 6.2; buffers like sodium phosphate prevent acid buildup, supporting yields of 12 g/L. CHO cells, producing antibodies such as atezolizumab, require a narrow pH range of 7.0–7.2 to preserve glycosylation patterns. Deviations can cut yields by 25%. Automated pH control using agents like sodium hydroxide ensures stability and consistent productivity across systems.
(iii) Dissolved Oxygen (DO): DO drives aerobic metabolism much like oxygen sustains a marathon runner. E. coli requires 30–50% DO saturation for optimal enzyme production; lower levels trigger anaerobic pathways, reducing yields by up to 20%. Pichia pastoris, used for methanol-induced insulin expression, needs around 40% DO, demanding tight aeration control. In Chlorella vulgaris photobioreactors, CO₂ sparging balances internal oxygen buildup, supporting consistent lipid yields. For Bacillus subtilis, DO sensors help maintain oxygen above 20% to prevent sporulation, which otherwise reduces α-amylase production.
(iv) Agitation and Aeration: Agitation and aeration work in tandem to deliver oxygen and nutrients, much like stirring a pot ensures even mixing. In Saccharomyces cerevisiae bioreactors, impellers typically operate at 200–500 rpm with aeration at 1.5 vvm (volume of air per volume of liquid per minute) to support ethanol production. CHO cells, which are shear-sensitive, require gentle agitation (50–100 rpm) in airlift bioreactors to sustain antibody yields around 3 g/L. Lactobacillus casei thrives under low aeration (0.5 vvm) to preserve microaerophilic conditions and maximize lactic acid output. For Chlorella vulgaris, mild bubbling in photobioreactors ensures effective CO₂ delivery without causing cell damage, supporting lipid accumulation. These parameters must be carefully balanced to maintain cell viability and optimize productivity, avoiding losses from shear stress or oxygen limitation.
Online Sensors and Process Control Systems
Maintaining optimal conditions in a bioreactor is like piloting a spacecraft; continuous monitoring and precise course corrections are essential to stay on target. Just as astronauts rely on real-time telemetry to navigate space, bioprocess engineers depend on online sensors and advanced process control systems to monitor key parameters such as pH, temperature, dissolved oxygen, CO₂, and agitation speed. These sensors act as the nervous system of the bioprocess, feeding data to automated control loops that adjust gas flow rates, nutrient feeds, or stirring speed to maintain ideal conditions. This tight feedback control ensures consistent cell performance, maximizes product yields, and minimizes the risk of contamination or metabolic drift. In high-value production systems, such as those used for therapeutic proteins or biofuels, even small deviations can lead to significant losses, making real-time bioreactor control a cornerstone of industrial biotechnology.
Online sensors and process control systems serve as the central intelligence of modern bioreactors, enabling precise and responsive control over critical parameters that directly impact productivity. Online sensors function like a dashboard of real-time gauges, continuously feeding data to guide bioprocess optimization. For instance, pH probes in E. coli bioreactors monitor acidity in real time and automatically trigger sodium hydroxide dosing to maintain pH at 7.0, boosting enzyme yields by up to 10%. Dissolved oxygen (DO) sensors using polarographic or optical electrodes monitor oxygen saturation in Pichia pastoris cultures, ensuring DO levels stay at 40% to support methanol-driven insulin production. In Bacillus subtilis fermentations, temperature probes maintain a stable 30°C, preventing heat stress that could suppress α-amylase expression.
Advanced tools, such as near-infrared (NIR) and Raman spectroscopy , allow non-invasive, real-time glucose monitoring in Saccharomyces cerevisiae fermentations, enabling fine-tuned feed rates to sustain ethanol production at 55 g/L. In phototrophic systems like Chlorella vulgaris, light sensors track photon flux density, maintaining levels near 2000 µmol/m²/s to support lipid biosynthesis, improving yields by approximately 15%. These sensors not only detect fluctuations but also enable proactive interventions before productivity drops occur.
Process control systems translate sensor data into intelligent action. Proportional-integral-derivative (PID) controllers serve as the autopilot of bioreactors, adjusting parameters like aeration or agitation in real time. In CHO cell cultures, PID controllers modulate air flow and mix to maintain 40% DO saturation, ensuring monoclonal antibody titers reach 3 g/L. Supervisory Control and Data Acquisition (SCADA) systems integrate sensor feedback from pH, temperature, DO, and agitation in Lactobacillus casei fermentations, reducing variability in lactic acid production to under 5%. These platforms not only automate corrections but also record detailed logs for regulatory oversight (Figure 10.7).

Figure 10.7. Bioreactor vessel and control sensors. The left panel shows an industrial-scale fermentation system equipped with precise control systems using PID algorithms. The right panel illustrates various sensors integrated into a stirred-tank bioreactor, including probes for pH, dissolved oxygen, temperature, and foam detection, which collectively enable real-time monitoring and control of the bioprocess. Image created with Google Gemini. Released under CC BY 4.0
Artificial intelligence (AI) is transforming process control into biomanufacturing by enabling smarter, faster, and more adaptive operations. Traditional systems depend on fixed control loops and static setpoints, but AI-driven platforms particularly those using machine learning (ML) can analyze continuous streams of real-time sensor data to detect subtle trends, predict process deviations, and optimize parameters dynamically. In fed-batch fermentations, machine learning (ML) models trained on historical glucose profiles can predict substrate depletion and adjust feeding rates with millisecond precision, preventing metabolic overflow and improving yields. Reinforcement learning algorithms further enhance control by fine-tuning PID systems in real time, adapting to fluctuations in temperature, pH, or dissolved oxygen without human intervention.
In mammalian cell cultures, AI platforms integrate spectrometric, imaging, and metabolic data to predict cell viability and productivity in advance, enabling preventive interventions that increase monoclonal antibody titers. In algal systems, AI regulates light exposure and CO₂ supply to maximize lipid production while minimizing energy use. These approaches also improve digital twin simulations of bioprocesses by continuously synchronizing virtual models with real-time data, allowing rapid scenario testing and optimization. Incorporating AI into bioproduction control systems improves consistency, shortens development timelines, and reduces batch-to-batch variability. For example, predictive models for Saccharomyces cerevisiae fermentations can anticipate glucose depletion and dynamically adjust feeding schedules, yielding a 12% increase in ethanol production. Similarly, in Chlorella vulgaris cultivation, supervisory control and data acquisition (SCADA) systems integrated with AI can regulate CO₂ sparging based on real-time biomass measurements, cutting energy consumption by 10% without compromising lipid accumulation. These intelligent systems significantly reduce human error, enhance reproducibility, and support regulatory compliance with FDA guidelines on process validation. In large-scale manufacturing, where even minor deviations can result in costly losses, AI’s ability to maintain tight control over complex bioprocess variables is essential for delivering high-quality, scalable, and economically viable production.
10.8. Fermentation and Cultivation Strategies
Fermentation and cultivation strategies are like choosing the perfect recipe, tailored to the ingredients and desired outcome. In biomanufacturing, these approaches guide how bacteria, yeast, or algae grow to produce valuable products such as enzymes, therapeutics, or biofuels. Whether batch, fed-batch, continuous, solid-state, submerged, or algal cultivation, each method affects yield, cost, and scalability, where a wrong choice can reduce output by up to 30% or increase expenses. This section explores these diverse strategies, showing how they optimize cell performance for specific applications. With practical examples and biotech insights, we’ll reveal how these methods power the production of everything from probiotics to sustainable biopolymers, ensuring cells perform at their best in the bioreactor. Fermentation strategies act like different cooking styles, each with its own pace and purpose. Batch, fed-batch, and continuous fermentation offer distinct ways to manage growth and product formation, customized for each organism and target compound.
(i) Batch Fermentation: This is like baking a cake all at once, adding all ingredients at the start and letting the process run to completion. For example, a 1000-liter stirred-tank bioreactor cultivating E. coli for recombinant human insulin is loaded with glucose (20 g/L) and ammonium sulfate (2 g/L), achieving insulin yields of 2 g/L in 24 hours. Its simplicity makes batch fermentation suitable for high-value products like Streptomyces venezuelae chloramphenicol, with yields around 0.5 g/L. However, nutrient depletion and waste buildup, such as acetate accumulation in E. coli, can reduce yields by up to 15%. Batch systems are cost-effective for small-scale production but less efficient for extended runs.
(ii) Fed-Batch Fermentation: This process is like gradually adding ingredients to a simmering stew, extending productivity by carefully controlling nutrient supply. In Pichia pastoris cultures producing antibodies, glucose or methanol is fed incrementally (e.g., 1 g/L/hr) to sustain AOX1-driven expression, boosting yields to 7 g/L in 5000-liter bioreactors over 48 hours. This method avoids substrate inhibition that can lower Saccharomyces cerevisiae ethanol yields by 20% in batch mode, enabling fed-batch ethanol production of 60 g/L. Similarly, CHO cells producing monoclonal antibodies like nivolumab use fed-batch with amino acid feeding to reach 3.5 g/L in 14 days. Figure 10.8a illustrates this process, showing precise glucose feed control in fed-batch bioreactors that enhance antibody yields while preventing inhibition. This approach is essential for scaling from lab to industrial production, balancing high yields with moderate complexity, making it ideal for pharmaceuticals and other high-value bioproducts.
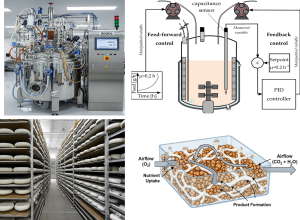
Figure 10.8a. Industrial fed-batch bioreactor and solid-state fermentation setup. Left: Fed-batch system with controlled nutrient addition to enhance microbial growth and bioproduct yields (e.g., antibodies, biofuels). Right: Solid-state fermentation for fungal mycelium production using sterilized substrates incubated under sterile, aerated conditions. Image created with Google Gemini. Released under CC BY 4.0.
(iii) Continuous Fermentation: This process is like a conveyor belt restaurant, with a steady inflow of ingredients and outflow of products. For example, Lactobacillus delbrueckii produces lactic acid at 15 g/L in a 10,000-liter chemostat, where fresh media is added and product removed at 0.1 L/hr to maintain steady-state growth. This method suits stable, high-volume products such as Zymomonas mobilis bioethanol, achieving 50 g/L with 30% lower energy costs than batch processes. However, contamination risks increase and, if not controlled with sterile filtration, yields can drop by 25%. Continuous fermentation is ideal for commodity products but demands advanced control systems to sustain stability. The choice between solid-state and submerged fermentation is like deciding whether to bake bread on a tray or simmer soup in a pot; each method fits different organisms and product types.
(iv) Solid-State Fermentation (SSF): The SSF process utilizes solid substrates with low moisture, such as a garden bed, to support microbial growth. For example, Ganoderma lucidum produces medicinal mycelium on sterilized lignocellulosic biomass pellets in tray bioreactors, reducing water usage by 80% compared to submerged fermentation. Other SSF applications include:
- Aspergillus niger produces citric acid at 25 g/kg on sugarcane bagasse trays, saving significant water.
- Streptomyces clavuligerus synthesizes clavulanic acid (1 g/kg) on wheat bran, a common agro-waste.
- Trichoderma harzianum grown on rice husks, yielding 12 g/kg cellulase for biofuels; rotating drum bioreactors ensure uniform aeration.
- Rhizopus oryzae produces lactic acid on soybean hulls, achieving 18 g/kg under optimized moisture (65%) and temperature (30°C).
- Penicillium chrysogenum generates penicillin precursors on corn steep liquor, reducing energy input by 15% through ambient temperature processing.
SSF systems typically incur 20% lower equipment costs due to their simpler, compact design, but require precise moisture control (60–70%) to prevent nutrient stratification, which can lower yields by up to 10%.
(v) Submerged Fermentation (SmF): immerses microbial cells in liquid media, much like fish swimming in a nutrient-rich aquarium. For example, Bacillus coagulans produces up to 15 g/L of lactic acid in stirred-tank bioreactors using 30 g/L glucose at pH 6.0, ideal conditions for bioplastic production. The uniform mixing in SmF supports high-density cultures; Pichia pastoris, for instance, can produce insulin at yields of 7 g/L in 5,000-liter fermenters. SmF is particularly suitable for fast-growing microorganisms like bacteria and yeast, but demands energy-intensive agitation (e.g., 500 rpm) and aeration (1.5 vvm), increasing operational costs by approximately 15% compared to solid-state fermentation (SSF). Yarrowia lipolytica is cultivated via SmF to produce omega-3 fatty acids, achieving 1.8 g/L in 1,000-liter bioreactors under tightly controlled oxygen levels (40% saturation) to optimize lipid biosynthesis. Despite higher energy demands, SmF remains the dominant platform for large-scale biomanufacturing due to its scalability, process control, and product consistency.
Algal Cultivation Strategies
Algal cultivation resembles farming a solar-powered crop, utilizing sunlight and carbon dioxide to generate biomass and synthesize valuable compounds. Cultivation methods for microalgae such as Chlorella vulgaris and Haematococcus pluvialis are optimized to match their photosynthetic behavior, requiring careful integration of light intensity, nutrient availability, and system design (Figure 10.8b).
(i) Open Pond Systems: Open Pond systems function like low-cost, open-air gardens, simple and economical, but vulnerable to environmental fluctuations and contamination. Widely used strains such as Chlorella vulgaris and Nannochloropsis gaditana thrive in raceway ponds supplemented with approximately 0.5 g/L nitrate, achieving protein yields around 1 g/L for use in dietary supplements (Figure 10.8b). Operating costs average $0.20 per liter, roughly 50% lower than those of closed systems. However, these systems are susceptible to contamination from airborne microbes or wild algal species, which can reduce productivity by up to 20%. Paddle wheels facilitate circulation, maintaining uniform light exposure and sustaining biomass concentrations near 0.5 g/L dry weight. Alkaliphilic species such as Spirulina platensis are cultivated at pH 9.5 to suppress invasive organisms, yielding approximately 0.8 g/L protein, making this approach suitable for affordable nutraceutical production.
(ii) Closed Photobioreactors (PBR): PBRs are akin to controlled-environment greenhouses engineered for precision and sterility. These systems enable consistent, high-quality production of specialty compounds. For instance, Haematococcus pluvialis accumulates astaxanthin, a potent antioxidant, at levels up to 0.1 g/L in tubular PBRs operated under high light intensity (2,500 µmol/m²/s) and enriched with CO₂ at 0.2 vvm. Tubular reactors may be oriented vertically or horizontally (Figure 8b,c), with configurations chosen to optimize light penetration and surface-area-to-volume ratios. Contamination is reduced by over 90%, ensuring high product purity essential for applications in cosmetics and pharmaceuticals. Likewise, Nannochloropsis gaditana cultivated in flat-panel PBRs can yield 1.5 g/L of omega-3 fatty acids. Automated regulation of light and temperature enhances production efficiency by up to 25% compared to open ponds, although operational costs, particularly for lighting and cooling, are typically 30% higher.
(iii) Hybrid Systems: Hybrid cultivation systems integrate the advantages of both open and closed approaches, initiating growth in sterile photobioreactors to generate a dense, uncontaminated inoculum, then transitioning to open ponds for large-scale biomass production. For example, Dunaliella salina is used to produce beta-carotene at concentrations around 0.2 g/L, beginning with closed-system cultivation to achieve cell densities of ~10⁶ cells/mL, followed by outdoor scale-up. This two-phase strategy cuts production costs by approximately 15% while maintaining compound integrity. Similarly, nutrient management, such as supplying 1 g/L nitrate for Chlorella vulgaris, can enhance biomass output by 10%. Hybrid systems offer a balanced compromise between purity, scalability, and economic feasibility, supporting a wide range of applications from biofuels to health-promoting products.

Figure 10.8b. Two major photobioreactors are used to produce phototrophic organisms in industry. They are: (a) raceway pond system, (b) tubular system, and (c) recirculating airlift system. Image created with Google Gemini. Released under CC BY 4.0
10.9. Process Monitoring and Automation
Monitoring a bioprocess is like checking the pulse of a living system. Real-time data and rapid response are vital to keep it healthy and productive. In biomanufacturing, tracking parameters such as cell density, nutrient concentration, and pH ensures organisms like E. coli or CHO cells perform optimally, delivering high yields of enzymes, therapeutics, or biofuels. Even a small oversight, such as delayed pH adjustment or nutrient depletion, can slash yields by 25% or compromise product quality. This section explores how in-line and at-line measurement tools, integrated with supervisory control and data acquisition (SCADA) systems, enable real-time monitoring and precise control. Like navigating a spacecraft through turbulence, automated feedback loops help steer bioprocesses safely toward high-efficiency production. With fresh examples and smart sensors, we’ll show how automation transforms the complexity of bioprocessing into reliable, scalable manufacturing from probiotics to bioplastics.
In-Line Measurements
These measurements take place directly inside the bioreactor and provide continuous insights without interrupting the process, much like a doctor monitoring a patient’s vital signs during surgery. Sensors embedded in the bioprocess system track critical variables such as pH, dissolved oxygen (DO), temperature, and biomass in real time. In E. coli cultures engineered to produce recombinant lipases for industrial detergents, in-line pH probes maintain values between 6.8 and 7.2 to prevent acid buildup that could reduce yields by up to 15%, allowing production of about 1.8 g/L. Oxygen sensors, typically based on Clark electrodes, are used in Pichia pastoris fermentations to maintain 30% oxygen saturation, supporting AOX1-regulated antibody expression at titers of 6 g/L. Near-infrared (NIR) spectroscopy monitors glucose concentrations in Saccharomyces cerevisiae during ethanol production, enabling precise feeding strategies to consistently achieve 50 g/L. Capacitance-based biomass sensors measure viable cell density in Lactobacillus rhamnosus probiotic fermentations, ensuring counts of 10⁹ CFU/mL. These in-line systems reduce response time by up to 80% compared to manual sampling, a major advantage in high-density cultures where conditions can shift quickly and unpredictably.
At-Line Measurements
Small samples can be withdrawn from the bioreactor and analyzed nearby, much like drawing blood for immediate testing in a clinic. These at-line techniques provide higher resolution and accuracy for parameters that are often difficult to measure effectively with in-line sensors. For example, in CHO cell cultures producing monoclonal antibodies such as durvalumab, high-performance liquid chromatography (HPLC) is performed every 6 hours to quantify antibody titers and ensure consistent yields of about 3 g/L. In fermentations with Bacillus amyloliquefaciens, α-amylase concentration is measured using at-line enzymatic assays to determine the optimal harvest time and maximize yields up to 2 g/L. In photobioreactors cultivating Chlorella zofingiensis, flow cytometry is used to assess cell viability, ensuring more than 95% live cells, critical for high-quality astaxanthin production at 0.15 g/L. Similarly, at-line gas chromatography in Zymomonas mobilis ethanol fermentations detects volatile by-products such as acetaldehyde or acetic acid, which can reduce yields by up to 10% if uncontrolled.
Although at-line methods typically take 10–30 minutes per analysis, their precision is essential for monitoring critical quality attributes, particularly in pharmaceutical bioprocesses where regulatory compliance (e.g., FDA) requires rigorous control. Combined with in-line systems, at-line measurements form a powerful hybrid monitoring platform. For instance, in Streptomyces lividans fermentations for antibiotic production, in-line dissolved oxygen (DO) sensors maintain 40% saturation, while PCR assays performed every 12 hours detect contaminant DNA, preventing contamination-related losses that could exceed $80,000 per batch. Together, these complementary approaches provide both rapid feedback and deep analytical insight, enhancing process safety and improving product consistency by up to 20%.
Supervisory Control and Data Acquisition (SCADA) Systems
SCADA systems act as the central nervous system of bioprocessing, much like a flight control tower that coordinates every movement to keep flights on schedule. They integrate sensor data, automate real-time adjustments, and log performance metrics to ensure that bioreactors of all sizes operate efficiently and consistently. SCADA collects data from in-line sensors, analyzes it, and adjusts parameters on the fly, similar to a chef fine-tuning a recipe as it cooks. In E. coli lipase production, SCADA monitors pH and dissolved oxygen (DO), automatically triggering sodium hydroxide additions or aeration adjustments to maintain optimal conditions, which can increase yields by 10%. In Pichia pastoris antibody fermentations, SCADA regulates methanol feeding (1 g/L/h) to sustain AOX1 promoter activity and achieve titers of 6 g/L. For CHO cell cultures, SCADA modulates temperature (37 °C) and CO₂ (5%) to maintain antibody production at 3 g/L with only 5% variability. In phototrophic cultures of Chlorella zofingiensis, SCADA controls light intensity (2000 µmol/m²/s) and CO₂ sparging to maximize astaxanthin yields. Beyond control, SCADA logs every parameter to ensure traceability and regulatory compliance, enabling Saccharomyces cerevisiae ethanol batches to consistently meet quality standards. These intelligent systems not only stabilize processes but also improve productivity and support compliance in industrial-scale bioproduction.
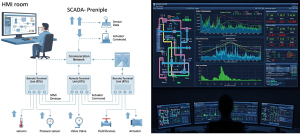
Figure 10.9 This schematic of a Supervisory Control and Data Acquisition (SCADA) system illustrates how sensor data from field devices is collected by Remote Terminal Units (RTUs) and sent to a central Human-Machine Interface (HMI) in the control room. The operator uses the HMI’s main display to view the industrial process layout, real-time data, and advanced control algorithms like predictive and PID control. This centralized interface enables continuous monitoring, automated control, and efficient management of complex operations. Image created with Google Gemini. Released under CC BY 4.0
Advanced Control Algorithms
SCADA systems employ advanced algorithms, such as proportional-integral-derivative (PID) controllers, to fine-tune bioprocess parameters in real time. For example, in Bacillus amyloliquefaciens fermentations, PID controllers regulate agitation (400 rpm) and aeration (1.5 vvm) to stabilize α-amylase production. When integrated with machine learning, SCADA capabilities are further enhanced. Predictive models can anticipate glucose depletion in Zymomonas mobilis cultures and enable dynamic feed adjustments that increase ethanol yields by 12%, reaching 50 g/L. In probiotic Lactobacillus rhamnosus production, SCADA systems combine real-time sensor inputs with at-line HPLC data to optimize nutrient delivery and maintain consistent yields of 10⁹ CFU/mL. These intelligent control strategies reduce manual intervention by 25%, minimize human error, and improve process scalability, key advantages in modern industrial biomanufacturing.
Benefits and Challenges
SCADA systems significantly enhance process efficiency. In Saccharomyces cerevisiae fermentations, optimized aeration via SCADA reduces energy consumption by up to 15%. Remote monitoring is especially critical in large-scale operations, for instance, 20,000-liter CHO cell bioreactors producing therapeutic antibodies can be monitored in real time, allowing operators to respond to deviations within minutes. However, the adoption of SCADA involves challenges: high initial costs (around $50,000 for a 5,000-liter setup), the need for trained personnel, and cybersecurity risks due to network connectivity. Secure data protocols and encryption are essential to mitigate threats. Despite these obstacles, the integration of real-time analytics, automation, and historical data logging makes SCADA systems indispensable for reproducible, high-performance biomanufacturing from recombinant proteins and antibodies to biofuels and probiotics.
10.10. Conclusion
Upstream processing forms the foundation of every biomanufacturing system. From the careful choice of microbial or mammalian hosts to the design of media, inoculum, and bioreactors, each decision directly shapes the performance of the process and the quality of the final product. The strategies outlined in this chapter, whether fed-batch cultivation of yeast, perfusion cultures of CHO cells, or the integration of agricultural by-products into media, illustrate how biological insight and engineering design come together to build efficient production systems. What stands out across these examples is the degree of control required. Small deviations in temperature, pH, or nutrient balance can cut yields dramatically, while effective sterilization, monitoring, and automation safeguard both consistency and scale. The result is not simply more products, but safer medicines, more affordable fuels, and sustainable enzymes that reach global markets. Ultimately, upstream processing is not a single step but a coordinated framework. Its principles enable the reliable transformation of living systems into biofactories, making possible the transition from laboratory innovation to industrial reality. The continued refinement of strains, bioreactors, and control systems will determine how quickly biomanufacturing can respond to emerging health needs, energy demands, and environmental challenges.
Recommended Reading Materials
- OpenStax Biology 2e: https://openstax.org/details/books/biology-2e
- Wikimedia Commons for microbial biotechnology images: https://commons.wikimedia.org/w/ index.php?search=microbial+biotechnology
Resources:
- Shuler, M. L., & Kargi, F. (2017). Bioprocess Engineering: Basic Concepts. Prentice Hall.
- Walsh, G. (2018). Bioprocess Engineering. Wiley.
- Seider, W. D., et al. (2017). Product and Process Design Principles. Wiley.
- Rosano, G. L., & Ceccarelli, E. A. (2014). Recombinant protein expression in E. coli: Advances and challenges. Frontiers in Microbiology, 5, 172.
- Nielsen, J. (2013). Metabolic Engineering. Springer.
- Zhang, J., et al. (2018). CRISPR/Cas9 for microbial strain engineering. Microbial Cell Factories, 17, 137.
- Wlaschin, K. F., & Hu, W. S. (2006). Fed-batch culture and media optimization. Advances in Biochemical Engineering/Biotechnology, 101, 43–74.
- Singh, V., et al. (2017). Media optimization for bioprocesses. Current Developments in Biotechnology and Bioengineering, 125–150.
- Lee, J., et al. (2005). Optimization of culture media for large-scale production. Biotechnology Progress, 21(5), 1441–1450.
- Stanbury, P. F., et al. (2016). Principles of Fermentation Technology. Elsevier.
- Sinclair, A., & Monge, M. (2005). Aseptic processing in biomanufacturing. BioPharm International, 18(9), 26–34.
- Flickinger, M. C. (2010). Sterilization techniques in industrial biotechnology. Encyclopedia of Industrial Biotechnology, 7, 4567–4589.
- El-Mansi, E. M. T., et al. (2007). Fermentation Microbiology and Biotechnology. CRC Press.
- Dunn, I. J., et al. (2003). Biological Reaction Engineering. Wiley-VCH.
- Doran, P. M. (2013). Bioprocess Engineering Principles. Academic Press.
- Villadsen, J., et al. (2011). Bioreaction Engineering Principles. Springer.
- Mandenius, C. F. (2016). Bioreactors: Design, Operation and Novel Applications. Wiley-VCH.
- Boudreau, E. D., & Martin, W. R. (2005). Environmental Control in Fermentation. BioProcess International.
- Glassey, J., et al. (2011). Process Analytical Technology in Bioprocessing. Advances in Biochemical Engineering/Biotechnology.
- Luttmann, R., et al. (2012). Automation in Bioprocessing. Wiley-VCH.

