11 Chapter 11: Downstream Bioprocess
Hemen Hosseinzadeh and Venkatesh Balan
11.1 Introduction to Downstream Processing
11.2 Cell Harvesting and Solid-Liquid Separation
11.6 Product Concentration and Polishing
11.7 Analytical Tools for Quality Control
11.8 Scale-up and Process Validation
11.9 Waste Management and By-product Valorization
11.1 Introduction to Downstream Processing
Learning Outcomes
- Explain the principles, goals, and economic impact of downstream processing in biomanufacturing.
- Describe core operations, including harvesting, cell disruption, extraction, purification, concentration, and polishing.
- Evaluate analytical tools, scale-up strategies, and validation requirements for consistent product quality.
- Assess sustainability approaches through waste management and by-product valorization.
- Integrate upstream and downstream steps to design efficient, scalable, and regulatory-compliant processes.
Downstream processing can be thought of as the clean-up crew after a big event, carefully sorting through the mixture to isolate and refine the valuable products. In bioproduction, this critical stage ensures that proteins, enzymes, chemicals, biofuels, and other products are efficiently extracted, purified, and polished to meet the stringent standards required for pharmaceuticals, food, or industrial applications. For example, when Escherichia coli produces insulin in a bioreactor, upstream processing generates raw broth, but it is the downstream processing that transforms it into the pure, injectable drug that saves lives. This phase is often the most resource-intensive, accounting for 50 to 80% of total production costs, and it directly determines whether a product is safe, effective, and commercially viable. At its core, downstream processing involves two key tasks: extraction, where the product is separated from the bioreactor broth, and purification, where it is refined to meet stringent quality requirements. By combining science, strategy, and economics, downstream processing enables the recovery of diverse high-value products ranging from therapeutic proteins and antibodies to sustainable polymers, industrial enzymes, chemicals, and biofuels, ensuring they are safe, effective, and ready for their intended application.
11.1.1. Recovery of products
Downstream processing is the clean-up stage in bioproduction, where complex mixtures are separated to isolate and refine valuable products. In a biorefinery, this step is especially critical because multiple streams such as biofuels, biochemicals, proteins, enzymes, and polymers are produced from renewable biomass or microbial systems. Efficient recovery ensures these products meet the strict standards required for pharmaceutical, food, industrial, and energy applications. Recovery typically involves two main tasks: extraction, separating the product from the biomass or fermentation broth, and purification, refining it to the required level of purity. For example, when Escherichia coli produces recombinant insulin, centrifugation at 5000 g can separate cells from the broth, recovering ~90% of insulin at 2 g/L. For intracellular products like Bacillus subtilis producing PHB for bioplastics, high-pressure homogenization at 1000 bars releases ~1.5 g/L of PHB. Similarly, microfiltration with 0.45 µm membranes can recover antibodies such as trastuzumab from Pichia pastoris at ~5 g/L, while flocculation with chitosan aggregates Chlorella vulgaris biomass to recover lipids for biofuels at ~1 g/L with 85% efficiency.
Because recovery can account for 50 80% of production costs, technologies such as filtration, chromatography, distillation, solvent extraction, and crystallization are widely used to improve yield and efficiency. In modern biorefineries, the aim is not just to recover a single product, but to generate multiple value streams in a cost-effective and sustainable way. Ultimately, product recovery is the critical step that transforms raw biological material into market-ready solutions from therapeutic proteins and industrial enzymes to renewable chemicals and biofuels, ensuring they are safe, effective, and commercially viable.
11.1.2. Purification of products
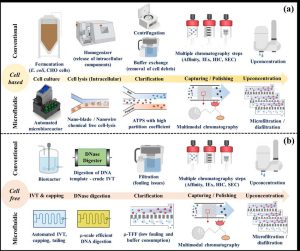
Figure 11.1. Simplified diagram illustrating the shift from conventional to microfluidic and continuous purification systems, encompassing cell-based production of proteins, antibodies, plasmid DNA, and cell-free synthesis of mRNA therapeutics. CC BY-SA 4.0, adapted from Wikimedia Commons (https://pubs.rsc.org/en/content/articlehtml/2024/lc/d3lc01097j).
Purification of Bioproducts is a critical stage of downstream processing where recovered products are refined to remove impurities such as host-cell proteins, nucleic acids, endotoxins, and metabolites. This step ensures that products meet the strict quality, safety, and regulatory standards required for pharmaceutical, food, and industrial applications. While recovery isolates the product from the bulk mixture, purification enhances its quality, with success measured in terms of yield (amount retained) and purity (freedom from contaminants).
Several purification techniques are commonly used depending on the product. Chromatography methods, such as ion-exchange for insulin or affinity for monoclonal antibodies, can achieve purities above 99% but often involve trade-offs in yield. Precipitation methods, like calcium hydroxide treatment of citric acid, and membrane-based approaches, such as ultrafiltration for antibiotics, are effective alternatives. However, each additional purification step can result in 5 10% product loss, making process design a balance between maximizing purity and maintaining sufficient yield.
Emerging trends in purification focus on advanced continuous and microfluidic systems, which improve efficiency and reduce losses compared to traditional batch methods. These innovations are particularly valuable for cell-based products (e.g., insulin, antibodies) and newer cell-free products (e.g., mRNA therapeutics). Ultimately, purification can account for up to 80% of total production costs, making it the most resource-intensive stage of bioprocessing. By integrating recovery and purification strategies with careful economic and engineering considerations, biorefineries can deliver safe, effective, and commercially viable products from pharmaceuticals and food additives to probiotics, biofuels, and sustainable materials.
11.1.3. Economics of the Process
Downstream processing (DSP) is one of the most expensive stages in biomanufacturing, accounting for 50 80% of total production costs due to equipment, reagents, consumables, and labor. High-value biologics like CHO cell-derived antibodies rely on costly methods such as Protein A chromatography, where resin can cost $10,000 per liter and add up to ~$20 per gram of purified antibody. In contrast, commodity products require simpler, cheaper steps; Aspergillus niger citric acid is purified through precipitation at just $0.50/kg, while Chlorella vulgaris lipid recovery via flocculation costs $0.20/L, 90% less than centrifugation, making such processes competitive in food or energy markets.
Facility, labor, and scale further shape DSP economics. Pharmaceutical-grade cleanrooms for Pichia pastoris insulin purification cost ~$500/m² annually, while trained staff add to ongoing expenses. Even small yield improvements have major impacts, for example, a 10% gain in E. coli insulin recovery can save $50,000 per batch. Scale also plays a decisive role: Streptomyces fradiae neomycin costs ~$5/g at 100-L scale but drops to ~$1/g at 10,000 liters, showing how commodity products demand cost-efficient processes, while high-value therapeutics can tolerate higher DSP costs.
Emerging strategies help reduce costs and improve efficiency. Single-use systems cut cleaning and validation costs by ~30% but raise consumable expenses, while membrane separations, aqueous two-phase systems (ATPS), and continuous chromatography offer higher yields with lower costs. Integrated biorefineries also improve economics by recovering multiple products such as fuels, enzymes, and chemicals from the same feedstock. Ultimately, success depends on balancing yield, purity, and scalability, with future innovations focused on intensification, modular systems, and sustainable separation technologies.
11.1.4. Process Integration
Integrating upstream and downstream processing in biorefineries is essential for achieving efficiency, cost reduction, and sustainability. Upstream choices directly shape downstream requirements: for example, high-density Pichia pastoris cultures reduce broth volume and centrifugation costs for antibody recovery, while secretion tags in E. coli simplify insulin recovery by avoiding costly lysis steps. Similarly, optimizing Chlorella vulgaris cultivation with low-cost nitrogen sources lowers the intensity of lipid purification, translating into measurable savings. These strategies highlight how thoughtful design at the microbial and media level can streamline subsequent recovery operations.
Process integration also extends to unit operations and system design. Hybrid separation technologies, such as combining centrifugation and microfiltration for Bacillus subtilis PHB recovery, minimize handling steps and shorten processing time. Continuous operations bring further benefits: membrane-based extraction for Aspergillus niger citric acid production sustains high yields while lowering labor costs, and perfusion bioreactors linked with continuous chromatography in CHO antibody manufacturing enhance throughput by 30%, albeit with higher upfront investment. Such approaches demonstrate how unit operation synergy and continuous processing drive both productivity and cost-effectiveness.
Advanced monitoring and resource integration strengthen this holistic framework. Process analytical technology (PAT), such as in-line HPLC monitoring of recombinant proteins, enables real-time quality control and reduces purification steps. Digitalization and machine learning provide predictive control over issues like membrane fouling and feeding strategies, ensuring consistent performance. At the same time, energy and water recycling, solvent recovery, and valorization of side streams like lignin or residual biomass enhance circularity and reduce environmental burdens. Together, these strategies ensure biorefineries are not only technically robust but also economically competitive and environmentally sustainable, enabling the scalable production of both high-value therapeutics and bulk bioproducts.
11.2 Cell Harvesting and Solid-Liquid Separation
Cell harvesting and solid-liquid separation form the foundation of downstream processing, serving as the very first step in transforming complex bioreactor broth into usable products. Much like sorting through a bustling farmer’s market to pick only the ripest fruits and leaving the stems and leaves behind, this stage involves separating valuable products such as enzymes, antibodies, lipids, or biofuels from a mixture of cells, growth media, and residual debris. Whether the product is extracellular, freely floating in the medium, or intracellular, trapped within the cells, the efficiency of this step directly impacts both yield and cost-effectiveness. A poorly executed separation can lead to up to 20% product loss or clog downstream purification equipment, underscoring its importance for both small-scale labs and industrial biorefineries.
The core techniques for cell harvesting and broth clarification include centrifugation, filtration, and flocculation, each chosen based on the properties of the biomass, the product location, and the process scale. Centrifugation, the workhorse of biomanufacturing, relies on centrifugal force to pellet cells away from the liquid, and is particularly useful for bacterial or yeast cultures. Filtration, whether microfiltration, ultrafiltration, or membrane-based crossflow systems, offers continuous operation and scalability, making it popular in large-scale protein recovery. Flocculation, by contrast, uses chemicals or polymers to aggregate suspended solids into larger clumps, simplifying their removal while reducing energy requirements. The choice of method often balances precision, scalability, and economics, ensuring that the product is separated without compromising quality.
Beyond the choice of technique, cell harvesting must also account for product stability, process economics, and integration with subsequent purification steps. For intracellular products, additional steps like cell disruption (via homogenization, sonication, or enzymatic lysis) are needed after harvesting to release the target molecules, whereas extracellular products are more directly recovered from clarified broth. Advances in continuous bioprocessing now allow harvesting and clarification to be integrated seamlessly with downstream purification, reducing bottlenecks and improving consistency. Ultimately, cell harvesting and solid-liquid separation act as the strainer in the bioprocess kitchen, removing what’s unnecessary, protecting what’s valuable, and ensuring the success of downstream recovery for medicines, food additives, sustainable polymers, and biofuels.
11.2.1. Centrifugation
Centrifugation is like spinning a salad dryer to fling water off lettuce, using high-speed rotation to separate solids from liquids based on density. For E. coli producing recombinant amylase for starch processing, continuous disc-stack centrifugation at 8000 g separates cells from the broth, recovering 90% of the extracellular enzyme at 1.5 grams per liter in 5000-liter batches. The high g-force ensures rapid separation, taking 30 minutes per batch, but energy costs can reach $3 per liter for large-scale operations. For P. pastoris secreting antibodies like cetuximab, centrifugation at 6000 g clarifies the supernatant, retaining 5 grams per liter of product with 95% efficiency. In Chlorella vulgaris cultures for biofuel lipids, lower speeds (4000 g) prevent cell lysis, recovering 0.8 grams per liter of biomass. Centrifugation is fast and effective, but requires robust equipment, adding 15% to downstream costs.
11.2.2. Filtration
Filtration functions much like a coffee filter, retaining solids while allowing liquids to pass through, making it a cornerstone of solid-liquid separation in bioprocessing. Microfiltration with 0.2-micron membranes is commonly employed to clarify S. cerevisiae broth after ethanol fermentation, achieving recovery of around 50 grams per liter of ethanol with up to 98% clarity for downstream distillation. For Bacillus licheniformis producing extracellular proteases, depth filtration with cellulose-based filters efficiently removes cellular debris, yielding about 2 grams per liter of enzyme. Tangential flow filtration (TFF), in which liquid flows parallel to the membrane surface, is widely used in mammalian systems such as CHO cell cultures producing therapeutic antibodies like ipilimumab, enabling product concentration up to 3 grams per liter while minimizing fouling, which otherwise can reduce throughput by as much as 10%. Beyond these, ultrafiltration and nanofiltration provide additional layers of refinement, concentrating proteins, recovering viral vectors, or fractionating metabolites based on molecular weight cut-offs. For instance, ultrafiltration membranes with 10 30 kDa cut-offs are frequently applied to concentrate monoclonal antibodies or to retain enzymes while allowing salts and small molecules to pass through. These techniques not only improve recovery but also reduce downstream purification burdens, lowering process time and buffer consumption.
Filtration is gentler than centrifugation, making it especially valuable for shear-sensitive cells or fragile macromolecules. However, challenges remain, including membrane fouling, pressure drops, and the high replacement costs of consumables. Fouling alone can increase production costs by more than $1 per liter in large-scale operations, underscoring the need for optimized membrane selection, backflushing strategies, or integration of flocculation pretreatments to reduce load. Advances such as single-use filtration systems, high-flux membranes, and continuous filtration platforms are helping to address these limitations, making filtration an increasingly central player in modern biomanufacturing.
Filtration is frequently integrated with centrifugation to achieve complete clarification of the broth. After centrifugation, pellets the bulk biomass, filtration removes finer debris and suspended solids, yielding a clarified liquid stream that is better suited for downstream purification. Figure 11.2 shows a typical setup in which a bioreactor harvest stream is processed through a centrifuge and subsequently passed through a filtration unit. This combined approach improves recovery efficiency, protects downstream equipment from fouling, and ensures higher product quality.
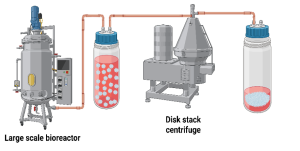
Figure 11.2. Centrifugation and filtration system for harvesting cells from bioprocessing broth. Centrifugation separates biomass from the supernatant, while filtration removes residual debris and further clarifies the broth, improving recovery of proteins, lipids, and other products. Created in https://BioRender.com.
11.2.3. Flocculation
Flocculation is akin to using a magnet to gather iron filings, causing cells or particles to aggregate and simplifying their separation from the medium. In Chlorella vulgaris lipid production, adding chitosan at 0.1 g/L induces cell clumping, settling 85% of the biomass, and enabling recovery of 1 g/L of lipids in 1,000-liter photobioreactors. This approach reduces energy costs by roughly 70% compared to centrifugation. Similarly, in Lactobacillus brevis cultures producing lactic acid for bioplastics, alum flocculation at 0.5 g/L aggregates cells, recovering around 10 g/L of product. Flocculation is cost-effective, typically around $0.2 per liter, but residual chemicals may require subsequent filtration or washing steps to prevent interference in downstream purification.
These techniques complement other solid-liquid separation methods such as centrifugation and filtration. For example, E. coli producing extracellular amylase can achieve 1.5 g/L recovery using a combination of high-speed centrifugation followed by membrane filtration. By integrating flocculation with these steps, operators can reduce energy consumption, minimize membrane fouling, and improve overall throughput. Choosing the appropriate method depends on whether the target product is intracellular, requiring cell disruption, or extracellular, which can be harvested directly from the clarified broth. Ultimately, centrifugation, filtration, and flocculation are tailored to the bioprocess, balancing speed, cost, yield, and product integrity. Their combined application sets the stage for downstream purification, ensuring that valuable products from enzymes and antibodies to lipids and organic acids are efficiently recovered at high quality. These separation strategies, when optimized, underpin the economic viability and scalability of biomanufacturing operations, highlighting their central role in the success of both small-scale and industrial bioprocesses.
Flocculation can be better understood by following the sequence illustrated in Figure 11.3. When a flocculant is added to a cell suspension, charged polymers or chemicals neutralize surface charges on the cells, causing them to aggregate into larger clumps. These aggregates then settle more readily by gravity or can be removed through filtration, significantly reducing the energy required for harvesting compared to centrifugation. The figure highlights each step of the process, from dispersed cells in the bioreactor broth to flocculant addition, clump formation, and final removal, providing a clear view of how this simple yet effective technique streamlines solid-liquid separation.
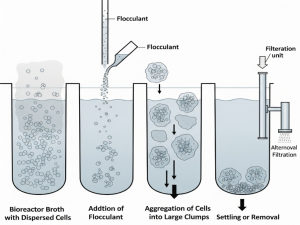
Figure 11.3. Flocculation process for cell harvesting. The addition of a flocculant causes suspended cells to aggregate into larger clumps, which settle naturally or are removed by filtration, reducing energy requirements and simplifying recovery compared to centrifugation. Image created with Google Gemini and released under CC BY 4.0.
11.2.4. Extracellular Products
Extracellular products, secreted into the culture broth, are like apples already picked, ready for separation from cells and debris. This simplifies recovery compared to intracellular products, which require cell disruption. For example, Pichia pastoris producing insulin under the AOX1 promoter secretes the protein into the medium, and centrifugation at 6,000 × g can recover up to 95% of the 5 g/L yield in the clarified supernatant. Similarly, Saccharomyces cerevisiae secretes ethanol during fermentation, which can be clarified using microfiltration with 0.45-micron membranes, achieving 98% recovery of 50 g/L ethanol for downstream distillation. Aspergillus oryzae secretes alpha amylase for food applications, and depth filtration can recover 2 g/L, although improper pH control (e.g., outside 6.0) may lead to protein aggregation and up to 5% product loss. While extracellular recovery avoids costly cell lysis, the typically diluted nature of culture broths (1.5 g/L for many proteins) necessitates concentration steps such as ultrafiltration or tangential flow filtration. These steps increase operational costs by approximately 10% but are critical for improving downstream purification efficiency and reducing storage and transport volumes. Integration with downstream chromatography or precipitation steps can further enhance recovery, minimize product loss, and streamline processing for large-scale production.
Scale and process design also impact extracellular extraction. Continuous clarification using membrane systems allows high-throughput processing of mammalian cell culture supernatants, while batch centrifugation remains common for microbial systems. Optimizing parameters such as feeding flow, pressure, and pH is essential to maximize yield while preserving product stability and bioactivity. Ultimately, efficient extracellular extraction sets the stage for subsequent purification, ensuring that proteins, enzymes, biofuels, and other secreted metabolites are recovered with high yield, quality, and economic viability.
11.2.5. Intracellular Products
Intracellular products, locked inside cells, are like walnuts that must be cracked open, requiring cell disruption before the product can be recovered. For instance, E. coli producing polyhydroxyalkanoate (PHA) for bioplastics is lysed using high-pressure homogenization at 1,200 bars, releasing 1.2 g/L of PHA, followed by centrifugation to recover approximately 90% of the product. Chlorella vulgaris, which stores lipids intracellularly, is disrupted via bead milling at 2,000 rpm, enabling solvent extraction and recovery of about 1 g/L of lipids. Similarly, Bacillus licheniformis, producing intracellular enzymes such as nattokinase, requires ultrasonication, followed by filtration, to recover 1.5 g/L.
Disruption methods must be carefully controlled to prevent product degradation; enzymes may lose up to 10% of activity during homogenization, so parameters like temperature (kept below 20°C), pressure, and residence time are critical. Alternative disruption strategies such as enzymatic lysis, osmotic shock, or chemical permeabilization can be employed depending on cell type, product sensitivity, and scale. For large-scale operations, continuous homogenizers or high-throughput bead mills improve processing efficiency and reduce energy costs compared to batch methods. Intracellular recovery is inherently more complex and can increase processing costs by 20% or more, but it is often justified by higher intracellular product concentrations, sometimes exceeding 10 g/L. Integration with downstream purification steps, such as ultrafiltration or chromatography, is essential to maximize overall yield and maintain product quality. Optimizing disruption techniques, recovery efficiency, and process flow ensures that intracellular products from bioplastics and lipids to enzymes and therapeutic proteins are extracted efficiently, safely, and cost-effectively at both lab and industrial scales.
11.2.6. Challenges and Efficiencies
Extracellular products, secreted into the culture broth, are like apples already picked, ready for harvesting without breaking open the cells. This simplifies recovery and reduces processing costs compared to intracellular products. For example, Pichia pastoris secreting insulin under the AOX1 promoter can achieve 5 g/L yields, and centrifugation at 6,000 × g recovers up to 95% of the protein from the clarified supernatant. Saccharomyces cerevisiae secretes ethanol during fermentation, which can be clarified with microfiltration using 0.45-micron membranes, recovering 50 g/L of ethanol with 98% efficiency for downstream distillation. Aspergillus oryzae produces extracellular alpha-amylase, which can be efficiently harvested by depth filtration, yielding around 2 g/L, although improper pH or temperature control can reduce yield by 5% due to protein aggregation.
While extracellular recovery avoids costly cell disruption, the often diluted nature of the broth (typically 1 5 g/L for proteins or metabolites) necessitates concentration steps such as ultrafiltration, tangential flow filtration, or adsorption to resins. These steps improve downstream purification efficiency, reduce storage and transport volumes, and minimize energy usage in subsequent operations. Integration with downstream chromatography, precipitation, or crystallization steps further enhances recovery and product quality while reducing losses.
Scale and process design are critical for extracellular product extraction. Continuous clarification with membrane systems or flocculation pretreatments allows high-throughput handling of mammalian or microbial culture supernatants, while batch centrifugation remains common for smaller operations. Optimizing parameters such as feed flow rate, membrane pore size, and pH ensures maximal recovery while preserving bioactivity and stability. Efficient extracellular extraction not only lowers production costs and energy consumption but also sets the foundation for high-quality downstream purification, enabling industrial-scale production of proteins, enzymes, biofuels, and other secreted bioproducts.
11.3 Cell Disruption Methods
Cell disruption is like cracking open a piñata to access the treasures inside, releasing valuable products trapped within cells during biomanufacturing. Intracellular compounds, whether enzymes from E. coli, lipids from Chlorella vulgaris, or biopolymers from Bacillus subtilis, must be efficiently liberated before they can be purified and formulated for use. This step is crucial across applications, from pharmaceuticals and therapeutic proteins to biofuels and food additives, because an improperly chosen method can degrade up to 15% of the product or substantially increase processing costs. Mechanical disruption techniques, such as high-pressure homogenization and bead milling, are widely employed for industrial-scale operations, providing consistent cell breakage and high throughput. Gentler, non-mechanical approaches, including chemical, enzymatic, or osmotic shock, are used for sensitive products or smaller-scale processes, helping preserve bioactivity while minimizing energy input. Selecting the optimal method depends on cell type, product stability, and process scale, with key parameters such as pressure, shear, temperature, and residence time carefully controlled to maximize recovery. Effective cell disruption is tightly integrated with downstream separation, such as centrifugation, filtration, or flocculation, to promptly recover the released product and prevent degradation. Optimized disruption strategies enhance yield, reduce energy consumption, and support scalability, ensuring that intracellular products from life-saving therapeutics to sustainable bioplastics and biofuels move efficiently from the bioreactor to market-ready form.
Figure 11.4 provides an overview of the major categories of cell lysis methods used in bioprocessing. Mechanical approaches, such as bead milling and high-pressure homogenization, rely on physical forces to achieve rapid and scalable cell breakage. In contrast, non-mechanical approaches are divided into physical, chemical, and biological methods, each offering gentler alternatives for sensitive products. Physical methods include heat, osmotic shock, and cavitation; chemical methods rely on detergents or alkalis to solubilize membranes, and biological methods use enzymes to selectively degrade cell walls. Presenting these methods side by side highlights the trade-offs in disruption efficiency, product stability, scalability, and cost, and helps illustrate why no single technique is universally optimal.
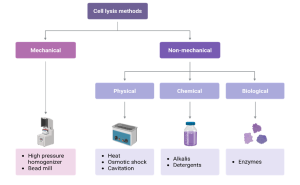 Figure 11.4. Overview of cell disruption methods. Mechanical approaches, such as high-pressure homogenization and bead milling, rely on physical forces, while non-mechanical approaches include physical, chemical, and enzymatic methods that provide gentler alternatives for sensitive products. Lugano, G. (2025) BioRender.
Figure 11.4. Overview of cell disruption methods. Mechanical approaches, such as high-pressure homogenization and bead milling, rely on physical forces, while non-mechanical approaches include physical, chemical, and enzymatic methods that provide gentler alternatives for sensitive products. Lugano, G. (2025) BioRender.
11.3.1. Mechanical Cell Disruption
Mechanical disruption methods are like using a sledgehammer to crack open a walnut, applying controlled physical force to break cell walls and liberate intracellular contents. Techniques such as high-pressure homogenization and bead milling are robust, fast, and highly scalable, but they must be carefully optimized to prevent damage to sensitive products and maintain bioactivity.
(i) High Pressure Homogenization (HPH): In this method, cells are forced through a narrow valve at extreme pressures, like squeezing toothpaste through a tiny nozzle, physically shearing cell walls and releasing intracellular contents. For E. coli producing human growth hormone, homogenization at 1,500 bar disrupts up to 95% of cells, yielding 1.8 g/L of protein in 1,000-liter batches. Each cycle takes approximately 20 minutes, and multiple passes are often required to achieve complete lysis, increasing energy consumption by ~$2 per liter. Similarly, Bacillus subtilis producing polyhydroxybutyrate (PHB) for bioplastics can be lysed at 1,200 bars, achieving 90% cell disruption and recovering 1.5 g/L of PHB.
Temperature control is critical: cooling systems maintain the broth below 20°C to prevent denaturation of sensitive proteins, which can otherwise reduce yields by 10%. While HPH is highly effective for bacteria and yeast, it produces fine cell debris that can complicate downstream filtration and centrifugation, sometimes reducing clarification efficiency by 10 15%. Despite these challenges, homogenization is highly scalable, making it suitable for large-volume industrial processes, though maintenance and wear on valves and seals can add ~10% to operational costs. Integration with downstream steps is essential for efficiency. Homogenized broth is typically immediately clarified via centrifugation, microfiltration, or flocculation to recover the product and minimize degradation. Advances such as continuous homogenizers and multi-stage systems help reduce energy usage, processing time, and overall cost per unit product, making HPH a preferred method for high-value proteins, enzymes, and biopolymers at industrial scales.
(ii) Bead Milling: Bead milling uses high-speed agitation of tiny glass or ceramic beads to mechanically grind cells, much like a blender pulverizing fruit into a smoothie. For Chlorella vulgaris producing intracellular lipids for biofuels, bead milling at 2,500 rpm with 0.5-mm beads disrupts about 90% of cells, releasing 1.2 g/L of lipids in 500-liter systems. The process typically runs for 15 minutes and requires cooling to prevent lipid oxidation, which can reduce yields by up to 5%. Similarly, Saccharomyces cerevisiae producing beta-glucan for food supplements is processed with 1-mm beads, achieving 2 g/L recovery with 85% efficiency.
Bead milling is one of the most established and widely applied mechanical methods for large-scale cell disruption. It is particularly effective for organisms with tough cell walls, including algae, fungi, and many bacteria. In this technique, a suspension of cells is passed through a chamber packed with small glass or ceramic beads. As the shaft rotates, the impeller generates intense agitation, driving repeated collisions between beads and cells that rupture the walls and release intracellular content. A cooling jacket surrounds the chamber to dissipate heat produced during operation, helping preserve the integrity of heat-sensitive products.
This method offers high throughput, robustness, and consistent performance, but it is not without drawbacks. Bead wear contributes to consumable costs (about $1 per liter), and the resulting debris can clog downstream filters, often requiring additional clarification steps such as centrifugation. Heat generation also needs to be carefully controlled, as excessive temperatures can reduce product yield by up to 5%. Despite these challenges, bead milling remains a workhorse for industrial bioprocessing. For example, in Lactobacillus casei producing intracellular enzymes, combining bead milling with centrifugation boosted recovery to 1.5 g/L, though energy costs rose by approximately 20% compared to gentler non-mechanical methods. Figure 11.5 illustrates the bead milling process, showing how bead motion under impeller action creates shear zones where cells are fragmented and intracellular components are released.
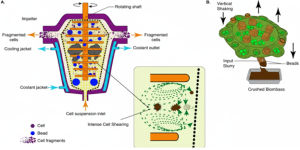
Figure 11.5. Bead milling for mechanical cell disruption. Cells are suspended in a bead-filled chamber and broken by shear forces from bead cell collisions, with a cooling jacket used to prevent overheating and product degradation. Image created with Google Gemini and released under CC BY 4.0.
11.3.2. Non-Mechanical Cell Disruption
Non-mechanical disruption methods act like carefully unlocking a treasure chest, gently releasing valuable intracellular products without applying harsh physical forces. Techniques such as chemical permeabilization, enzymatic lysis, and osmotic shock are milder than mechanical approaches, helping preserve product structure and biological activity. While these methods are often slower and less amenable to large-scale operations, they are particularly suited for sensitive cells or high-value products where maintaining integrity is critical. Non-mechanical methods preserve product quality, losing only 5% of activity compared to 10% in mechanical methods, but their slower speed and higher reagent costs limit industrial use. For L. casei enzymes, combining osmotic shock with enzymatic disruption achieves 80% recovery at 1.5 grams per liter, offering a compromise for sensitive products.
(i) Chemical Disruption: Chemical methods use detergents or solvents to solubilize cell membranes, much like dish soap breaking down grease, releasing intracellular products without harsh mechanical forces. For Pichia pastoris producing intracellular enzymes such as phytase for animal feed, 0.5% sodium dodecyl sulfate (SDS) disrupts approximately 80% of cells, yielding 1.2 g/L in 200-liter batches. The process runs at 25°C over 1 hour, minimizing protein degradation, but SDS residues must be removed through dialysis or filtration, adding around $0.50 per liter to costs. Similarly, Rhodococcus erythropolis producing cholesterol oxidase achieves 0.8 g/L recovery with 75% efficiency using 1% Triton X-100.
Chemical disruption is generally gentle on proteins, maintaining their activity, but is less effective for organisms with rigid or tough cell walls, such as algae. Additionally, disposal of chemical reagents contributes to environmental and regulatory costs, often increasing overall expenses by 10%. These methods are particularly suited for laboratory-scale operations or high-value products where preserving product stability outweighs throughput concerns.
(ii) Enzymatic Disruption: Enzymatic methods use specific enzymes, such as lysozyme or cellulase, to selectively degrade cell walls, much like a precise chisel carving stone, gently releasing intracellular products. For E. coli producing human interferon, lysozyme at 0.2 g/L disrupts about 85% of cells, yielding 1 g/L of protein in 100-liter systems. The process takes 2 hours at 30°C, preserving protein activity, though enzyme costs (around $5 per gram) add roughly $1 per liter to overall expenses. Similarly, Aspergillus niger, producing intracellular glucoamylase, uses 0.1 g/L cellulase to recover 1.5 g/L with 80% efficiency.
Enzymatic disruption is highly specific, minimizing damage to sensitive products and maintaining bioactivity, making it ideal for therapeutic proteins and high-value enzymes. However, slower reaction times and the cost of enzymes can limit scalability for large industrial operations. Combining enzymatic lysis with mild mechanical methods, such as low-pressure homogenization, can improve recovery as demonstrated in E. coli, where lysozyme plus gentle homogenization increases yield to 90%, balancing efficiency with cost. These methods are particularly useful when product stability is critical, or when downstream purification benefits from minimal debris, highlighting their role in integrated downstream processing strategies for pharmaceuticals, enzymes, and specialty biochemicals.
For microalgae such as Chlorella vulgaris, a purely mechanical or purely enzymatic approach often yields incomplete product release. A combined strategy is therefore employed. In this workflow, the biomass is first disrupted mechanically, producing a crude lysate that contains proteins, lipids, and carbohydrates. The lysate is then subjected to enzymatic treatment with lipase, which hydrolyzes membrane lipids and enhances the separation of intracellular components. Figure 11.6 shows how this two-step method leads to a clearer division of liquid and solid fractions, reducing downstream processing challenges. This hybrid approach not only improves recovery efficiency but also lowers the overall energy requirement compared to extended bead milling or homogenization alone.
(iii) Osmotic Shock: Osmotic shock exploits water movement across cell membranes, like soaking dried fruit to make it burst. For S. cerevisiae producing intracellular invertase, resuspending cells in a hypotonic solution (0.1 M NaCl) causes 70% cell lysis, releasing 1 gram per liter of enzyme in 50-liter batches. The process takes 3 hours and is low-cost ($0.2 per liter), but incomplete lysis reduces yields by 20% compared to mechanical methods. Chlorella vulgaris, producing antioxidant peptides, uses osmotic shock with distilled water, recovering 0.5 grams per liter with 65% efficiency, suitable for small-scale nutraceutical production. Osmotic shock is gentle and inexpensive but slow and less effective for robust cells, making it ideal for fragile or lab-scale applications.
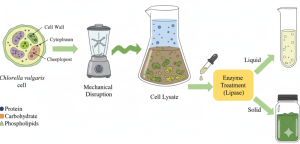
Figure 11.6. Combined disruption of Chlorella vulgaris using mechanical shear followed by enzymatic lysis. The initial shearing step generates a crude lysate, and subsequent lipase treatment hydrolyzes membrane lipids, releasing proteins and metabolites into a clarified liquid phase while residual biomass settles as a solid fraction. Image created with Google Gemini and released under CC BY 4.0.
11.4 Extraction Techniques
Compare the advantages, limitations, scalability, and cost-effectiveness of these methods for industrial applications. Extraction in bioprocessing is like panning for gold in a rushing river, carefully separating valuable product proteins, lipids, or antibiotics from a complex mixture of cells, media, and debris. After organisms such as E. coli or Chlorella vulgaris produce their target molecules, extraction techniques recover these products from the broth or disrupted cells, setting the stage for downstream purification. Choosing the wrong method can degrade up to 20% of the product or drastically increase costs, whereas an optimized approach ensures high recovery, purity, and cost-efficiency. Key extraction strategies include solvent extraction, aqueous two-phase extraction (ATPE), and supercritical CO₂ extraction. Solvent extraction efficiently isolates lipids and hydrophobic compounds from cell debris or algal biomass. ATPE leverages partitioning between immiscible aqueous phases to selectively extract proteins or enzymes with minimal denaturation. Supercritical CO₂ extraction provides a clean, tunable method for delicate bioactives, like essential oils or nutraceuticals, without leaving toxic residues. Each technique has trade-offs in throughput, scalability, and cost, making method selection critical for industrial bioprocess design. Figure 11.7 illustrates these methods side by side, highlighting their principles: partitioning into an organic solvent, selective distribution between aqueous phases, or using pressurized CO₂ for efficient, residue-free recovery. Visualizing the processes together clarifies why certain techniques are suited for bulk products like lipids or commodity chemicals, while others are ideal for sensitive therapeutic proteins or high-value nutraceuticals. These extraction strategies form a vital bridge between bioreactor output and market-ready products, ensuring both efficiency and quality in downstream processing.
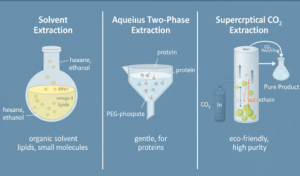
Figure 11.7. Illustration of three key extraction techniques in downstream bioprocessing. Solvent extraction uses organic solvents, such as hexane or ethanol, to isolate hydrophobic compounds, including lipids and small molecules. Aqueous two-phase extraction (ATPE) separates proteins into polymer–salt phases, maintaining biological activity while avoiding toxic solvents. Supercritical CO₂ extraction employs pressurized CO₂ to recover high-purity bioactives, such as astaxanthin, in an environmentally sustainable manner. Image created with Google Gemini and released under CC BY 4.0.
Solvent Extraction
Solvent extraction is like using a sponge to soak up oil from a spill, selectively pulling the desired product into a solvent based on its chemical properties. It’s widely used for its simplicity and effectiveness, especially for hydrophobic compounds like lipids or small molecules. Solvent extraction involves mixing a sample with an organic solvent, like hexane or ethanol, to dissolve the target product, followed by phase separation to recover it. For Nannochloropsis oculata producing omega-3 fatty acids for nutraceuticals, hexane extraction at a 1:4 biomass-to-solvent ratio recovers 1.5 grams per liter of lipids from cell debris in 1000-liter batches, achieving 90 percent efficiency in 2 hours. The solvent is evaporated, leaving lipids concentrated for further purification. Streptomyces rimosus produces oxytetracycline, an antibiotic, extracted with ethyl acetate, yielding 0.7 grams per liter with 85 percent recovery. The process is cost-effective, with solvent costs at $0.5 per liter, but requires ventilation systems to handle volatile fumes, adding 10 percent to operating costs. For S. cerevisiae producing squalene for cosmetics, chloroform-methanol extraction recovers 0.5 grams per liter, though solvent toxicity necessitates careful waste disposal, increasing environmental costs by 15 percent.
Advantages and Limitations
Solvent extraction is scalable and efficient for hydrophobic products, recovering up to 95 percent of lipids or antibiotics. Its low equipment costs make it ideal for bulk products like biofuels. However, solvent residues can contaminate products, requiring additional purification for Nannochloropsis oculata lipids to meet food-grade standards, adding $0.3 per liter. Flammable solvents like hexane also pose safety risks, and high solvent volumes can increase costs for low-concentration products. For Streptomyces rimosus antibiotics, integrating centrifugation before extraction reduces solvent use by 20 percent, improving efficiency. Solvent extraction shines for industrial-scale applications but requires careful handling to ensure product safety and environmental compliance.
Aqueous Two-Phase Extraction
Aqueous two-phase extraction (ATPE) is like sorting laundry into lights and darks, using two water-based phases to separate products based on their affinity. This gentle method is ideal for delicate biomolecules like proteins, avoiding harsh solvents.
Process and Applications
Aqueous two-phase extraction (ATPE) uses two immiscible aqueous phases, typically formed by mixing polymers such as polyethylene glycol (PEG) and dextran or salts such as ammonium sulfate. For Escherichia coli producing recombinant human interferon-alpha, a PEG–phosphate system at 10% w/v separates 1.2 g/L of protein into the PEG phase, recovering 90% within one hour. The process operates at 25 °C, maintaining protein activity, and is applied in 500-L pharmaceutical production systems. Bacillus amyloliquefaciens, which produces subtilisin, a detergent enzyme, uses a PEG–dextran system and achieves 1.5 g/L recovery with 85% efficiency. Chlorella vulgaris, which produces antioxidant peptides, uses a PEG–sulfate system to extract 0.6 g/L, avoiding organic solvents and ensuring food compatibility. Although ATPE costs approximately $1 per liter due to polymer expenses, it reduces purification steps by 15% compared to conventional chromatography.
Advantages and Limitations
ATPE is gentle, preserving protein structure, and avoids toxic solvents, making it ideal for high-value bioproducts like E. coli interferon-alpha. It’s scalable and integrates well with centrifugation, reducing processing time by 20 percent. However, phase-forming agents like PEG are expensive, and recycling them adds $0.5 per liter in costs. The method struggles with low-concentration products, as partitioning efficiency drops below 1 gram per liter, reducing yields by 10 percent. For B. amyloliquefaciens subtilisin, optimizing salt concentrations improves recovery to 90 percent. ATPE is a powerful tool for protein extraction, but it requires cost management for industrial applications.
Supercritical CO₂ Extraction
Supercritical CO₂ extraction is like using a futuristic vacuum to suck out treasures, leveraging CO₂’s unique properties at high pressure and temperature to extract products cleanly. This eco-friendly method is prized for its purity and sustainability, especially for high-value compounds.
Process and Applications
Supercritical CO₂ extraction operates above CO₂’s critical point (31°C, 73.8 bar), where it acts as both liquid and gas, dissolving products like a solvent. For Haematococcus pluvialis producing astaxanthin for cosmetics, supercritical CO₂ at 300 bar and 40°C extracts 0.2 grams per liter of antioxidant from dried biomass in 2 hours, achieving 95 percent purity. The process is used in 100-liter systems, with CO₂ recycled to reduce costs to $2 per liter. Y. lipolytica, producing squalene, uses supercritical CO₂ with ethanol as a co-solvent, recovering 0.6 grams per liter with 90 percent efficiency. For Penicillium chrysogenum, producing penicillin, supercritical CO₂ extracts 1 gram per liter from fermentation broth, avoiding organic solvents and ensuring pharmaceutical-grade purity. The process is energy-intensive, consuming 20 kWh per batch, but produces no solvent waste.
Advantages and Limitations
Supercritical CO₂ extraction is environmentally friendly, leaving no toxic residues, and achieves high purity, making it ideal for Haematococcus pluvialis astaxanthin in nutraceuticals. It’s effective for hydrophobic compounds and scalable with CO₂ recycling, reducing costs by 30 percent. However, high-pressure equipment costs $100,000 for a 500-liter system, and energy demands increase operating costs by 25 percent compared to solvent extraction. For Y. lipolytica squalene, co-solvents like ethanol improve yield but add $0.5 per liter. The method excels for high-value products but is less practical for low-cost commodities like biofuels due to capital costs.
Integration and Optimization
Combining extraction methods enhances efficiency. For Chlorella vulgaris peptides, ATPE followed by ultrafiltration boosts recovery to 0.7 grams per liter, reducing costs by 10 percent. For Nannochloropsis oculata lipids, solvent extraction paired with centrifugation minimizes solvent use, saving $0.2 per liter. Supercritical CO₂ extraction for Penicillium chrysogenum penicillin integrates with filtration to remove debris, improving yield by 15 percent. Process analytical technology (PAT), like in-line UV spectroscopy, monitors product partitioning in ATPE, optimizing phase ratios and boosting E. coli interferon-alpha recovery by 10 percent. These integrated approaches ensure high recovery, purity, and cost-effectiveness, tailoring extraction to the product’s needs, from antibiotics to sustainable lipids.
11.5 Purification Techniques
Solvent extraction is like using a sponge to soak up oil from a spill, selectively pulling the desired product into a solvent based on its chemical properties. It is widely used for its simplicity and effectiveness, particularly for hydrophobic compounds such as lipids or small molecules. The process involves mixing the biomass or fermentation broth with an organic solvent, like hexane, ethanol, or chloroform, which dissolves the target product, followed by phase separation to recover it. For example, Nannochloropsis oculata, producing omega-3 fatty acids for nutraceuticals, achieves 1.5 g/L lipid recovery in 1000-liter batches using hexane at a 1:4 biomass-to-solvent ratio, with 90% efficiency in 2 hours. The solvent is subsequently evaporated, concentrating the lipids for downstream purification. Similarly, Streptomyces rimosus produces oxytetracycline extracted with ethyl acetate, yielding 0.7 g/L with 85% recovery, while S. cerevisiae squalene is recovered via chloroform-methanol extraction at 0.5 g/L, though solvent toxicity necessitates careful waste handling.
Solvent extraction is scalable and highly efficient for hydrophobic products, often achieving up to 95% recovery for lipids and antibiotics. Its low equipment requirements make it attractive for bulk applications such as biofuels. However, solvent residues can contaminate products, requiring additional purification, e.g., N. oculata lipids may need extra steps to meet food-grade standards, adding ~$0.3 per liter. Flammable solvents like hexane pose safety risks, and high solvent volumes increase costs for low-concentration products. Pre-treatment steps such as centrifugation can improve efficiency, as seen with S. rimosus antibiotics, where separating cells before extraction reduced solvent use by 20%. Overall, solvent extraction is a versatile, cost-effective method for industrial-scale recovery of hydrophobic bioproducts, but it demands careful handling, environmental management, and integration with purification steps to ensure product quality and regulatory compliance. Its balance of simplicity, scalability, and efficiency makes it a cornerstone technique in bioprocessing, particularly for lipids, antibiotics, and other nonpolar compounds.
11.5.1. Aqueous Two-Phase Extraction (ATPE)
This method is like sorting laundry into lights and darks, using two water-based phases to selectively separate products based on their affinity. This gentle technique is particularly suited for sensitive biomolecules such as proteins, avoiding the harsh solvents used in traditional extraction. ATPE relies on forming two immiscible aqueous phases, typically by combining polymers like polyethylene glycol (PEG) with dextran or with salts such as ammonium sulfate. For example, Escherichia coli producing recombinant human interferon-alpha can be processed using a PEG phosphate system at 10% w/v, separating 1.2 g/L of protein into the PEG-rich phase with 90% recovery in just one hour. The process operates at 25°C, preserving protein activity, and is applicable at the 500-L scale in pharmaceutical production. Similarly, Bacillus amyloliquefaciens, producing subtilisin, a detergent enzyme, achieves 1.5 g/L recovery with 85% efficiency using a PEG dextran system, while Chlorella vulgaris, producing antioxidant peptides, can be extracted with a PEG sulfate system at 0.6 g/L, avoiding organic solvents and maintaining food-grade compatibility. ATPE is scalable and integrates well with upstream and downstream processes, such as centrifugation, reducing processing time by $1 per liter. and recycling expenses (~$0.5 per liter) can increase operational costs. Efficiency also declines with low-concentration products (<1 g/L), reducing yields by ~10%, though optimizing salt or polymer concentrations, such as for subtilisin, can improve recovery to 90%. Overall, ATPE is a powerful, mild, and environmentally friendly extraction strategy for high-value proteins, but cost management and process optimization are essential for industrial applications.
11.5.2. Supercritical CO₂ Extraction
This method is like using a futuristic vacuum to selectively pull out treasures, exploiting CO₂’s unique properties at high pressure and temperature to extract compounds cleanly and efficiently. This environmentally friendly method is prized for its high purity and sustainability, making it ideal for high-value products. Supercritical CO₂ operates above its critical point (31°C, 73.8 bar), where it behaves simultaneously as a liquid and gas, dissolving target molecules like a solvent while leaving behind solids.
For example, Haematococcus pluvialis, producing astaxanthin for cosmetics, can be processed at 300 bars and 40°C, extracting 0.2 g/L of antioxidant from dried biomass in 2 hours with 95% purity at the 100-L scale. Yarrowia lipolytica, producing squalene, uses supercritical CO₂ with ethanol as a co-solvent, recovering 0.6 g/L with 90% efficiency, while Penicillium chrysogenum, producing penicillin, achieves 1 g/L extraction from fermentation broth, avoiding toxic solvents and ensuring pharmaceutical-grade purity. CO₂ is typically recycled, reducing operating costs to ~$2 per liter, although energy demands can reach 20 kWh per batch. The method is highly effective for hydrophobic compounds, scalable, and produces no solvent residues, making it ideal for nutraceuticals like astaxanthin or high-value lipids such as squalene. Co-solvents can enhance yield but increase costs (e.g., ethanol adds $0.5 per liter). However, high-pressure equipment is capital-intensive $100,000 for a 500-L system, and energy requirements raise operating costs by ~25% compared to conventional solvent extraction. While supercritical CO₂ extraction excels for premium products, it is less suitable for low-cost commodities like bulk biofuels due to high upfront and operational costs.
11.5.3. Integration and Optimization
Combining extraction methods can significantly enhance efficiency and cost-effectiveness. For example, Chlorella vulgaris peptides processed with ATPE followed by ultrafiltration achieve 0.7 g/L recovery while reducing overall costs by 10%. In Nannochloropsis oculata lipid production, coupling solvent extraction with centrifugation minimizes solvent usage, saving $0.2 per liter. For Penicillium chrysogenum penicillin, integrating supercritical CO₂ extraction with filtration removes residual debris and improves yield by 15%. Advanced monitoring tools further optimize integrated extraction. Process Analytical Technology (PAT), such as in-line UV spectroscopy, tracks product partitioning in ATPE systems, allowing real-time adjustment of phase ratios and boosting E. coli interferon-alpha recovery by 10%. These integrated strategies tailor extraction to the specific product, whether high-value therapeutics, enzymes, or sustainable lipids. By combining methods, implementing real-time monitoring, and optimizing process parameters, bioprocessors can achieve higher recovery, improved purity, and greater cost-efficiency, bridging the gap between bioreactor output and market-ready products.
11.5 Purification Techniques
Purification in bioprocessing is like polishing a rough gemstone into a sparkling jewel, transforming crude bioreactor output into a pure, market-ready product. Whether it’s E. coli producing enzymes or Chlorella vulgaris yielding lipids, purification removes contaminants such as host-cell proteins, salts, nucleic acids, or cell debris to meet strict quality standards for pharmaceuticals, food additives, or biofuels. Even a small error can result in 10 15% product loss or failure to meet regulatory specifications, translating into significant economic consequences. This stage relies on core purification techniques, including precipitation and crystallization, chromatography (affinity, ion-exchange, size-exclusion), and membrane-based separations (ultrafiltration, nanofiltration). Precipitation and crystallization, for instance, are like sifting flour to remove lumps, selectively isolating the target compound from complex mixtures. These approaches are cost-effective and widely used for initial purification, particularly for small molecules, enzymes, and proteins. Chromatography and membrane-based methods provide higher resolution and specificity. Affinity chromatography isolates antibodies or other high-value proteins based on specific interactions, ion-exchange chromatography separates molecules by charge, and ultrafiltration concentrates products while removing low-molecular-weight impurities. Together, these methods refine bioproducts ranging from therapeutic proteins to sustainable biopolymers, ensuring purity, activity, and regulatory compliance while preparing them for downstream formulation and commercial distribution.
11.5.1. Precipitation
Precipitation in biorefining uses salt, pH adjustments, or solvents to render the target product insoluble, allowing it to separate from the bulk solution. This method is widely applied for the initial purification of proteins, organic acids, and other biochemicals due to its simplicity and scalability. For example, Aspergillus niger, producing citric acid for food applications, can use ammonium sulfate at 40% saturation to precipitate contaminating proteins, enabling recovery of 20 grams per liter of citric acid at ~90% purity in 1000-liter batches. The process typically takes about 1 hour, with an operating cost of $0.3 per liter, though residual salts necessitate an additional filtration or dialysis step, adding roughly $0.1 per liter. Optimizing parameters such as pH (6.0 for citric acid) or salt concentration can improve selectivity, increasing recovery by 5%.
Similarly, E. coli producing recombinant human albumin can use ethanol precipitation at 20% v/v, recovering approximately 2 grams per liter with 85% efficiency. Precipitation is particularly useful in biorefining bulk products like citric acid, amino acids, or polyhydroxyalkanoates, where gentle and cost-effective concentration is important. It is also commonly combined with downstream techniques such as centrifugation, microfiltration, or ultrafiltration to remove co-precipitated impurities and further enhance purity. While precipitation is robust and easily scaled, it has limitations. Non-specificity can cause co-precipitation of unwanted compounds, reducing overall yield by 5 10%. Additionally, solvent-based methods require careful handling and recovery to minimize environmental impact and process costs. Despite these challenges, precipitation remains a cornerstone in industrial biorefining, providing an economical way to concentrate products and reduce the load for subsequent purification steps, balancing efficiency, cost, and scalability.
11.5.2. Crystallization
Crystallization is a key downstream technique that refines bioproducts into pure, solid crystals, akin to sugar crystallizing from a cooling syrup. This method leverages solubility differences, temperature shifts, or pH changes to selectively precipitate the target compound while leaving impurities in solution. For example, Streptomyces griseus producing streptomycin can be processed by cooling the fermentation broth to 4°C and adjusting pH to 7.0, inducing crystallization, and recovering 0.8 grams per liter with 95% purity in 500-liter batches. The process typically takes 4 hours, minimizes solvent usage, and costs around $0.5 per liter, making it economical for high-value antibiotics.
Bacillus subtilis, producing riboflavin for vitamin supplements, uses crystallization at pH 5.0 to recover 1 gram per liter with 98% purity. Crystallization is highly selective, making it ideal for pharmaceuticals and nutraceuticals where regulatory standards demand high purity. However, it has limitations: slow kinetics can extend processing time, and product loss in the mother liquor can reduce overall yields by 10 15%. Process optimization, such as controlled cooling rates, seeding with crystal nuclei, and careful pH management, can enhance crystal size, purity, and recovery.
Crystallization is often integrated with other purification strategies to maximize efficiency. For instance, combining precipitation with crystallization for E. coli albumin can increase product purity to 99%, although overall recovery may drop to 80% due to multiple handling steps. Additionally, crystallization can be adapted to continuous systems or automated cooling platforms to improve scalability and reproducibility for industrial applications. By tuning solubility, temperature, and agitation, crystallization not only enhances product quality but also reduces downstream solvent and energy costs, making it a cornerstone technique in biorefining and high-value bioproduct manufacturing.
11.5.3. Chromatography-based purification
Chromatography is like organizing a library, where each book finds its place on the right shelf. In bioprocessing, it separates the target product from a complex mixture of impurities by exploiting differences in chemical properties, size, or binding affinity. This technique is essential for achieving high purity, making it invaluable for proteins, enzymes, and other sensitive biomolecules. Chromatography not only enhances product quality but also enables consistent, reproducible purification at industrial scales, bridging the gap between crude extracts and market-ready bioproducts.
(i) Affinity Chromatography: Affinity chromatography is like using a custom key to unlock a specific door, selectively capturing the target product from a complex mixture. For CHO cells producing monoclonal antibodies such as atezolizumab, Protein A columns bind the antibody’s Fc region, achieving recoveries of 3 g/L with 99% purity in 5000-liter batches. While the process is fast, typically 2 hours, the high cost of resin (~$10,000 per liter) adds about $15 per gram to production. Similarly, P. pastoris producing His-tagged insulin under the AOX1 promoter uses nickel-affinity chromatography (Ni-NTA) to recover 5 g/L of protein at 95% purity. Affinity chromatography is extremely selective, making it indispensable for high-value biologics and FDA-approved therapeutics. Resin longevity is a key factor; after approximately 50 cycles, degradation increases costs by 10%, but careful cleaning and reuse can cut expenses by 20%. The method’s precision ensures consistent quality, minimizing downstream polishing steps and reducing overall process variability. Figure 10.8 illustrates this approach, showing a Ni-NTA column capturing His-tagged insulin from P. pastoris and achieving 95% purity. The diagram highlights how selective binding simplifies purification, reduces contamination, and supports regulatory compliance. Visualizing the process underscores why affinity chromatography remains a cornerstone for producing high-purity bioproducts efficiently and cost-effectively.
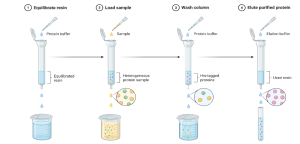
Figure 11.8. Diagram of affinity chromatography, illustrating a Ni-NTA column for purifying His-tagged proteins like insulin, enhancing bioproduct purity. Ona, S. (2025) BioRender.
(ii) Ion Exchange Chromatography: Ion exchange chromatography separates molecules based on their net charge, much like sorting charged marbles on a magnetized board. For E. coli producing recombinant lysozyme, anion exchange at pH 8.0 binds negatively charged proteins, achieving recoveries of 1.5 g/L with 90% purity in 1000-liter production systems. The process typically takes 1.5 hours and costs around $2 per liter, though non-specific binding can reduce yield by 5%. Similarly, Bacillus amyloliquefaciens producing subtilisin uses cation exchange at pH 6.0 to recover 1.8 g/L of enzyme efficiently.
Ion exchange chromatography is highly versatile, suitable for proteins, peptides, and other charged biomolecules, and is easily scaled from lab to industrial operations. Careful buffer selection and pH control are essential to maintain selectivity and prevent product loss, particularly for low-concentration or sensitive products. Combining ion exchange with pre-concentration steps, such as ultrafiltration, can further enhance yield and reduce buffer consumption. This method balances cost-effectiveness, scalability, and product purity, making it a cornerstone in industrial bioprocessing for enzymes, therapeutic proteins, and other high-value bioproducts. Its adaptability allows integration with other purification steps, including affinity or size-exclusion chromatography, to achieve final product specifications while minimizing losses and operational expenses.
(iii) Size Exclusion Chromatography: Size exclusion chromatography (SEC) separates molecules based on size, much like sifting marbles through a series of sieves. For S. cerevisiae producing recombinant human growth hormone, SEC recovers 1.2 g/L with 98% purity, efficiently removing smaller protein contaminants over 2 hours. The method is gentle, preserving protein structure and bioactivity, making it ideal for sensitive therapeutic proteins. Similarly, Aspergillus niger derived glucoamylase achieves 1.5 g/L recovery at 99% purity, meeting stringent food-grade enzyme standards.
While SEC excels in precision and selectivity, it has inherent limitations. Low throughput and high resin costs (approximately $5,000 per liter) restrict its use primarily to polishing steps rather than bulk purification. Product dilution during separation often necessitates subsequent concentration steps, such as ultrafiltration, adding to operational costs. Integrating SEC with preceding purification methods, such as ion exchange or affinity chromatography, enhances overall purity. For example, combining SEC with ion exchange for E. coli lysozyme increases purity to 99.5%, although overall yields may decline by ~10% due to multiple handling steps. Despite its cost and throughput constraints, SEC remains indispensable for achieving ultra-high purity, particularly for biologics, enzymes, and other high-value biomolecules. Its gentle separation preserves molecular integrity, reduces aggregation, and ensures compliance with regulatory standards. By carefully integrating SEC with upstream concentration and preceding chromatographic steps, industrial bioprocessing can achieve the optimal balance of yield, purity, and product quality.
11.5.4. Membrane Separation
This separation method acts like a fine mesh straining tea leaves from a brew, selectively separating products based on size or molecular characteristics. Techniques such as ultrafiltration and nanofiltration are widely employed to concentrate and purify proteins, enzymes, lipids, and other bioproducts. These methods are gentle, preserving biological activity, and can be easily scaled from laboratory to industrial operations. Membrane systems also allow continuous processing, reduce solvent use, and integrate well with upstream and downstream steps, enhancing overall process efficiency.
(i) Ultrafiltration: Ultrafiltration employs membranes with pore sizes typically ranging from 1 to 100 kDa to concentrate target proteins and remove small impurities. For CHO cell-derived antibodies such as durvalumab, ultrafiltration using a 30-kDa membrane concentrates the product to 3.5 g/L with 90% recovery in 5,000-liter batches. The process takes approximately 2 hours and costs around $1 per liter, though membrane fouling can lower throughput by 10%, necessitating periodic cleaning cycles. In P. pastoris producing insulin, ultrafiltration effectively removes salt and low-molecular-weight proteins, achieving 5 g/L with 95% recovery. Tangential flow filtration (TFF), where the flow runs parallel to the membrane surface, further reduces fouling and enhances throughput by 15%, as seen in B. subtilis riboflavin production at 1 g/L. Overall, ultrafiltration is a scalable and cost-efficient separation method, but routine maintenance and monitoring are essential to sustain performance and product quality.
(ii) Nanofiltration: Nanofiltration employs membranes with very small pores (0.1 1 kDa) to selectively remove ions, salts, and low-molecular-weight compounds, like filtering fine sediment from water. In Chlorella vulgaris, producing antioxidant peptides, nanofiltration at 0.5 kDa purifies 0.7 g/L with 90% purity, effectively removing salts to meet food-grade standards. The process requires about 3 hours and costs $1.5 per liter, with high-pressure operation (~20 bar) increasing energy consumption by 10%. For Streptomyces griseus producing streptomycin, nanofiltration removes residual media components, achieving 0.8 g/L at 95% purity. Nanofiltration is highly effective for small molecules and ions but is less suitable for large proteins, which can clog membranes and reduce efficiency by approximately 5%. Combining ultrafiltration with nanofiltration, for instance, in E. coli lysozyme production can achieve 99% purity while streamlining the purification process. Overall, nanofiltration is a versatile and precise method for polishing low-molecular-weight products and preparing them for final formulation or downstream applications.
11.5.5. Integration and Optimization
Integrating purification techniques enhances efficiency, yield, and cost-effectiveness. For CHO cell-derived antibodies, combining affinity chromatography with ultrafiltration streamlines the workflow, reducing processing steps and saving approximately $5 per gram of product. In Aspergillus niger citric acid production, pairing precipitation with nanofiltration decreases purification costs by 20% while maintaining product quality.
Advanced monitoring tools, such as process analytical technology (PAT) using in-line conductivity or UV sensors, allow real-time optimization of buffer conditions and process parameters. For example, PAT improves P. pastoris insulin recovery by 10%, minimizing product loss and reducing the need for repeated purification cycles. By strategically combining precipitation, crystallization, chromatography, and membrane separation, bioprocesses achieve high-purity outputs while balancing cost, throughput, and yield. This integrated approach ensures that a wide range of products from therapeutic proteins and enzymes to organic acids and sustainable biopolymers are efficiently purified to meet regulatory standards and market requirements.
11.6 Product Concentration and Polishing
11.6 Product Concentration and Polishing
Product concentration and polishing in bioprocessing are like adding the final brushstrokes to a masterpiece, transforming a nearly complete product into its market-ready form. After E. coli produces an enzyme or Chlorella vulgaris yields lipids, these products must be concentrated, refined, and stabilized to meet the stringent quality standards of pharmaceuticals, food additives, or biofuels. This stage is critical; errors can result in up to 10% product loss or failure to meet regulatory requirements, incurring significant costs in rework or waste. This section explores key techniques such as drying, spray drying, freeze-drying, evaporation, and distillation, and final formulation and stabilization, illustrating how each method enhances product quality, stability, and shelf life. Drying, for instance, is like gently baking out excess moisture from a sponge cake, concentrating on the product while preserving its integrity. Spray drying is ideal for heat-stable compounds like microbial enzymes or bioactive lipids, producing fine powders suitable for storage and downstream formulation. Freeze-drying, in contrast, preserves sensitive proteins, vaccines, and probiotics by sublimating water under low temperature and vacuum conditions. Evaporation and distillation further concentrate on products by removing solvents or water, making them compatible with formulation or packaging requirements. Final formulation and stabilization steps, including buffer optimization, excipient addition, and controlled storage conditions, ensure that products maintain activity, purity, and functionality during transport and shelf life. Together, these methods polish bioproducts from crude harvests into high-quality, stable materials, ready for industrial, medical, or consumer applications.
11.6.1. Spray Drying
Spray drying is like misting perfume into a warm breeze, atomizing a liquid product into fine droplets that dry almost instantly in a stream of hot air. For Lactobacillus acidophilus producing probiotics for dietary supplements, spray drying at an inlet temperature of 150°C concentrates cells to 10¹¹ CFU per gram while maintaining 90% viability in 1000-liter batches. The process occurs within seconds per droplet, costing about $0.50 per liter, though high temperatures can reduce cell survival by 5%, necessitating protective agents such as maltodextrin. Similarly, Aspergillus oryzae, producing alpha-amylase for food processing, can be spray-dried to yield 2 grams per kilogram of dry enzyme with 95% retained activity.
Spray drying is fast, scalable, and ideal for bulk products, but the energy required to heat the air adds roughly 15% to operational costs. Optimizing nozzle design, atomization pressure, and airflow for L. acidophilus can improve recovery by 10%, enhancing both efficiency and cost-effectiveness. The technique also allows precise control over particle size, moisture content, and powder density, which are critical for product stability and downstream formulation.
Figure 11.9 illustrates the spray-drying process, showing how atomized L. acidophilus droplets pass through the heated chamber and emerge as a stable, high-viability probiotic powder. The diagram emphasizes the importance of nozzle adjustments and airflow optimization in preserving cell viability and maximizing yield. Understanding this setup is essential for producing cost-effective, market-ready products with consistent quality.
Figure 11.9. Diagram of a spray drying system, illustrating atomization and drying chamber for concentrating probiotics and enzymes with high stability. Tucak-Smajić, A. (2025) BioRender.
11.6.2. Freeze Drying (Lyophilization)
This method is like preserving a delicate flower by freezing it and gently removing the ice while maintaining its structure and function. For Pichia pastoris producing recombinant hepatitis B vaccine, freeze-drying at 50 °C and 0.1 mbar preserves 1 g/L of antigen with 99% potency in 200-L systems. The process takes about 24 hours and costs around $2 per liter due to energy-intensive vacuum requirements, but it is exceptionally gentle, making it ideal for sensitive proteins. Similarly, Bacillus subtilis, which produces nattokinase for cardiovascular supplements, retains 1.5 g/L with 98% activity after freeze-drying. By combining protective excipients like trehalose with optimized drying cycles, costs for P. pastoris products can be reduced by 10% while maintaining quality and stability.
Freeze-drying extends product shelf life to up to two years, making it the method of choice for high-value biopharmaceuticals, though its slow pace and high cost limit widespread application. In contrast, spray drying suits robust, high-volume products like probiotics, achieving 90 99% recovery in seconds and offering greater scalability. Together, these drying techniques balance speed, cost, and product stability, allowing bioprocess engineers to tailor methods to product sensitivity and market needs.
11.6.3. Evaporation
Evaporation removes water using heat, concentrating on the target product much like reducing a broth to a rich, flavorful glaze. For Saccharomyces cerevisiae producing ethanol for biofuels, rotary evaporation at 60 °C concentrates 50 g/L of ethanol to 80% v/v in 5000-L systems, taking 2 hours and costing $0.3 per liter. The process is energy-efficient for volatile products but can degrade heat-sensitive compounds, reducing yields by 5% if cooling controls are not applied.
Aspergillus niger, producing citric acid for food additives, uses vacuum evaporation at 40 °C, concentrating 20 g/L to 50% w/v with 95% recovery. Evaporation is scalable and low-cost, but fouling of heat exchangers can increase maintenance requirements by 10%. Optimizing parameters such as vacuum pressure and flow rate, for example, for S. cerevisiae ethanol can improve throughput by 15% and enhance overall energy efficiency. Overall, evaporation is a versatile concentration method for liquid bioproducts, balancing cost, scalability, and product integrity, though careful control is essential to prevent degradation of heat-sensitive molecules.
11.6.4. Distillation
Evaporation and distillation are like simmering a sauce to concentrate flavors or distilling spirits to capture their essence, concentrating bioproducts by removing water or separating volatile compounds. These methods are essential for liquid bioproducts and small molecules, enabling downstream formulation, purification, or packaging while maintaining product quality and stability. Distillation separates compounds based on boiling points, akin to purifying essential oils from herbs. For Zymomonas mobilis producing bioethanol, fractional distillation at 78 °C isolates 95% pure ethanol at 50 g/L in 10,000-L systems, taking 3 hours and costing $0.4 per liter. The process is highly selective, crucial for fuel-grade ethanol, but energy-intensive, adding approximately 20% to operating costs. Similarly, Clostridium acetobutylicum produces butanol for industrial solvents, which is distilled at 117 °C, recovering 10 g/L with 90% purity. Distillation excels for small, volatile molecules but is unsuitable for proteins and other heat-sensitive products, which denature at elevated temperatures.
Integrating evaporation with distillation improves efficiency, as demonstrated with Z. mobilis ethanol, reducing energy consumption by 10% while maintaining 90 95% product recovery. Overall, these methods are robust for concentrating liquids and recovering volatile bioproducts at industrial scale, but careful energy management is critical to control costs and preserve product integrity, particularly for large-scale commodity bioprocesses.
11.6.5. Final Formulation and Stabilization
Final formulation and stabilization are like packaging a gourmet meal for delivery, ensuring the product remains safe, effective, and stable until it reaches the consumer. This step customizes the product’s form, incorporates stabilizers, and extends shelf life. Formulation prepares the product for its intended use, much like shaping dough into loaves before baking. For CHO cells producing monoclonal antibodies such as adalimumab, the purified protein (3 g/L) is formulated into a sterile, injectable solution using 50 mM phosphate buffer at pH 7.2. Aseptic filling in 100-L systems ensures FDA sterility compliance, costing approximately $5 per liter. Lactobacillus acidophilus probiotics are formulated into capsules at 10¹¹ CFU/g, blended with prebiotics like inulin to enhance gut delivery, at $1 per kilogram. Chlorella vulgaris antioxidant peptides are formulated into powders for dietary supplements, mixed with excipients such as cellulose, yielding 0.8 g/kg. Formulation ensures convenient and effective delivery, but excipient costs and regulatory testing can increase expenses by up to 15%.
Stabilization protects products from degradation, like sealing a jar of jam to maintain freshness. For the Pichia pastoris hepatitis B vaccine, adding 0.5% sucrose as a stabilizer extends shelf life to 3 years at 4 °C, retaining 99% potency. Bacillus subtilis nattokinase stabilizes with 1% glycerol, preventing enzyme aggregation and maintaining 1.5 g/L activity for 18 months. Saccharomyces cerevisiae ethanol is stabilized with antioxidants such as BHT to prevent oxidation during storage, maintaining 50 g/L quality for biofuel blending. Stabilization often involves lyophilization, controlled atmosphere packaging, or additive incorporation, increasing costs by approximately $0.5 per liter but preventing up to 10% product loss during storage. For high-value biopharmaceuticals like CHO cell antibodies, sterile filtration and nitrogen flushing during packaging further reduce microbial risk, ensuring compliance with USP standards. Together, formulation and stabilization convert purified bioproducts into safe, durable, and user-ready products suitable for pharmaceuticals, dietary supplements, and biofuels.
11.6.6. Integration and Optimization
Integrating concentration and polishing steps streamlines the process. For L. acidophilus probiotics, spray drying followed by capsule formulation reduces processing time by 20%, saving $0.2 per kilogram. For S. cerevisiae ethanol, evaporation paired with distillation minimizes energy costs by 15%. Process analytical technology (PAT), like in-line viscosity sensors, optimizes spray drying for Aspergillus oryzae alpha-amylase, improving yield by 10%. These techniques, drying, evaporation, distillation, formulation, and stabilization, work together to deliver concentrated, stable, high-quality products, balancing cost and performance for applications from biopharmaceuticals to sustainable fuels.
11.7 Analytical Tools for Quality Control
Quality control in bioprocessing is like being a detective at a crime scene, meticulously analyzing every clue to ensure that the final product, whether an enzyme, antibody, or biofuel, is pure, safe, and effective. When E. coli produces a recombinant protein or Chlorella vulgaris yields lipids, analytical tools such as spectroscopy, HPLC, GC-MS, and ELISA verify that the product meets strict standards for composition, potency, and purity. Even a single impurity like a trace contaminant or misfolded protein can compromise a pharmaceutical batch or reduce biofuel efficiency by 10%, resulting in substantial financial losses. This section explores these analytical techniques and their critical role in product characterization, purity assessment, and regulatory compliance, showing how they act as the “magnifying glass and fingerprint kit” of bioprocessing. Spectroscopy provides rapid identification of chemical structures, HPLC separates and quantifies complex mixtures, GC-MS detects volatile compounds with high sensitivity, and ELISA measures specific proteins or metabolites. Together, these tools ensure that bioproducts from life-saving vaccines to sustainable bio-based materials meet exacting quality standards before reaching the market. By integrating these analytical methods throughout production, manufacturers can monitor process consistency, detect deviations early, and optimize yields, safeguarding both product performance and regulatory compliance.
11.7.1. Spectroscopy
Spectroscopy is like shining a flashlight through a prism to reveal a product’s chemical signature, using light absorption, emission, or magnetic properties to analyze composition and structure. Ultraviolet-visible (UV-Vis) spectroscopy quantifies protein concentration in E. coli cultures producing recombinant lipases for detergents, measuring absorbance at 280 nm to confirm 1.5 grams per liter with 95% accuracy in 1000-liter batches. Near-infrared (NIR) spectroscopy monitors glucose levels in S. cerevisiae ethanol fermentations, ensuring 50 grams per liter yields by detecting real-time nutrient depletion. Fourier-transform infrared (FTIR) spectroscopy identifies lipid profiles in Nannochloropsis salina for biofuels, confirming 1.2 grams per liter of omega-3 fatty acids. Nuclear magnetic resonance (NMR) spectroscopy provides detailed structural information of metabolites and small molecules, allowing characterization of complex fermentation products. Fluorescence spectroscopy detects specific biomolecules via intrinsic or tagged fluorophores, useful for monitoring cofactors like NADH/NADPH or tracking protein expression. Spectroscopy is fast, often taking minutes, and non-destructive, costing around $0.2 $1 per sample, but requires careful calibration to avoid 5 to 10% variability in complex mixtures. Its versatility makes it ideal for in-line and at-line quality control, structural verification, and real-time process monitoring across bioprocessing applications. Other widely used spectroscopy methods are given below.
(i) Gas Chromatography Mass Spectrometry (GC-MS): GC-MS is like a molecular detective, identifying compounds by separating them as gases and then analyzing their unique mass “fingerprints.” Gas chromatography separates complex mixtures, while mass spectrometry provides precise molecular identification. For Clostridium acetobutylicum producing butanol for industrial solvents, GC-MS quantifies 10 g/L of butanol and detects trace acetone impurities at 0.1 g/L in 5000-liter batches. In Yarrowia lipolytica producing squalene for cosmetics, GC-MS verifies 0.5 g/L with 95% purity, ensuring residual lipids or contaminants are absent. Each analysis takes about 20 minutes per sample and costs roughly $2, factoring in helium and instrument expenses. GC-MS is exceptionally sensitive, detecting compounds at parts-per-billion levels, and regular calibration minimizes variability to 5%, making it indispensable for monitoring volatile and low-concentration bioproducts in bioprocessing.
(ii) Liquid Chromatography Mass Spectrometry (LC-MS): LC-MS is like a molecular detective for non-volatile and complex biomolecules, combining liquid chromatography to separate compounds in solutions with mass spectrometry for precise identification and quantification. It is widely used for proteins, peptides, metabolites, and small molecules. For example, in Pichia pastoris producing recombinant insulin, LC-MS confirms protein identity and purity, detecting modifications or impurities at microgram-per-liter levels. The method is highly sensitive and specific, with analysis times ranging from 10 to 60 minutes per sample, though instrument costs are high. LC-MS is essential for monitoring quality, stability, and post-translational modifications in bioprocessed products.
11.7.2. High-Performance Liquid Chromatography (HPLC)
HPLC is like sorting a deck of cards by suit and rank, separating molecules based on their interactions with a stationary phase and the mobile phase. For CHO cells producing monoclonal antibodies like pembrolizumab, reverse-phase HPLC quantifies antibody titers at 3 g/L with 99% purity, detecting impurities such as host cell proteins, aggregates, or degradation products in about 30 minutes. For Aspergillus niger producing citric acid for food additives, ion-exchange HPLC confirms 20 g/L with 98% purity, ensuring compliance with GRAS standards. HPLC’s high resolution and reproducibility are critical for pharmaceuticals, biopharmaceuticals, and nutraceuticals. Columns can cost $5,000, and sample preparation adds $1 per analysis; solvent usage also contributes to operational costs and environmental considerations. Optimizing mobile phase composition and gradients for CHO antibodies improves throughput by 15% and enhances peak resolution. HPLC also supports stability studies, kinetic analyses, and impurity profiling, making it indispensable for regulatory submissions and batch release. Figure 11.10 illustrates the HPLC column in action, separating pembrolizumab to confirm 99% purity and revealing minor impurities through retention times. This setup underscores HPLC’s vital role in ensuring product quality, consistency, and compliance with stringent standards.
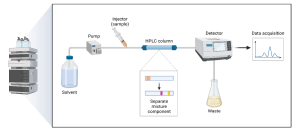
Figure 11.10. Diagram of an HPLC system, showing column separation for antibody purity testing. Huang, E. (2025) BioRender.
11.7.4. Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA is like a lock-and-key test, using antibodies to specifically detect and quantify target proteins with exceptional sensitivity. For Pichia pastoris producing recombinant insulin under the AOX1 promoter, ELISA measures 5 g/L of insulin with 99% specificity, detecting misfolded or degraded proteins as low as 0.01 g/L in 200-L systems. For Bacillus subtilis producing a vaccine antigen, ELISA confirms 1 g/L with 98% purity, ensuring immunogenicity for clinical applications. The assay typically takes 2 hours and costs $0.5 per sample, but its dependency on high-quality, specific antibodies limits its applicability to protein products. Automation of ELISA for P. pastoris insulin reduces analysis time by 20%, increases throughput, and ensures compliance with FDA and other regulatory standards. ELISA’s precision, sensitivity, and reproducibility make it a gold standard for protein quantification and quality control. In bioprocessing, product characterization and purity testing are like giving a final exam to the bioproduct, verifying identity, potency, and safety, and confirming that it is free from contaminants and performs as intended.
11.7.5. Product Characterization
Characterization verifies a bioproduct’s identity, structure, and functionality, like checking a passport for authenticity. For CHO cell-derived antibodies such as atezolizumab, mass spectrometry confirms molecular weight (150 kDa) and glycosylation patterns, ensuring 3 g/L meets therapeutic standards. In Saccharomyces cerevisiae ethanol production, GC-MS validates 50 g/L ethanol with 99% purity, confirming fuel-grade quality. For Nannochloropsis salina lipids, FTIR spectroscopy profiles fatty acids, ensuring 1.2 g/L of omega-3s suitable for nutraceuticals. Circular dichroism (CD) spectroscopy assesses protein folding in E. coli lipase, confirming 95% active conformation for detergent applications. Characterization ensures consistency, potency, and safety across batches, but advanced analyses like mass spectrometry are costly (~$5 per sample) and require skilled technicians. Combining HPLC with mass spectrometry for CHO antibodies reduces analysis time by 10% and enhances throughput, demonstrating how integrated approaches improve efficiency while maintaining rigorous quality standards.
11.7.6. Purity Testing
Purity testing ensures bioproducts are free from contaminants, like inspecting a diamond for flaws. For Pichia pastoris insulin, ELISA detects host cell proteins down to 0.01 g/L, confirming 99.9% purity suitable for pharmaceutical use. HPLC identifies minor impurities in Aspergillus niger citric acid, verifying 98% purity for food-grade applications. For Bacillus subtilis vaccine antigens, SDS-PAGE electrophoresis visualizes protein bands, confirming 98% purity and detecting aggregates that could reduce immunogenicity by 5%. In Clostridium acetobutylicum butanol production, GC-MS ensures acetone or ethanol contaminants remain below 0.1 g/L, meeting industrial standards. Purity testing is essential for regulatory compliance, but performing multiple assays can increase costs by ~$2 per sample. Automating HPLC for P. pastoris insulin reduces analysis time by 15%, improving throughput and efficiency in quality control.
11.7.7. Integration and Optimization
Combining complementary analytical methods strengthens quality control in bioprocessing. For CHO cell antibodies, HPLC paired with ELISA confirms 99.9% purity and proper antigen binding, ensuring compliance with FDA standards. For Nannochloropsis salina lipids, NIR spectroscopy alongside GC-MS verifies both lipid content and purity, reducing analysis costs by 10%. Process Analytical Technology (PAT), such as in-line UV-Vis spectroscopy, enables real-time monitoring of S. cerevisiae ethanol, optimizing distillation and saving $0.3 per liter. These integrated approaches provide a robust framework to guarantee that bioproducts from high-value therapeutics to industrial biofuels meet stringent quality standards while balancing accuracy, efficiency, and cost-effectiveness.
11.8 Scale-up and Process Validation
Scaling up and validating a bioprocess is like taking a recipe from a home kitchen to a large industrial bakery, ensuring every batch maintains the same quality while meeting strict safety standards. In biomanufacturing, transitioning from small-scale experiments to full-scale production requires meticulous planning to preserve product quality, yield, and safety for enzymes, antibodies, or biofuels. Errors during scale-up can reduce yields by 20% or trigger regulatory failures, resulting in costly rework. This section explores the nuances of pilot-scale versus industrial-scale production, emphasizing process reproducibility, regulatory compliance, and the strategies that ensure bioproducts meet global standards. Scaling up downstream processing is akin to upgrading from a bicycle to a high-speed train, necessitating adjustments to accommodate larger volumes without compromising performance. Pilot-scale operations 50 500 liters) serve as a critical bridge between lab-scale experiments and industrial-scale production (1,000 20,000 liters), each with unique technical and regulatory challenges. To illustrate this progression, Figure 11.11 depicts the pathway from laboratory experiments to pilot-scale optimization and finally to commercial production, highlighting how equipment size, process control, and regulatory oversight evolve at each stage. This visualization underscores the importance of reproducibility, validation, and compliance in delivering consistent, high-quality bioproducts from life-saving therapeutics to environmentally sustainable materials.
11.8.1. Pilot-Scale Considerations
Pilot-scale processing is like a dress rehearsal, providing a controlled environment to test the feasibility and optimization of downstream steps before full-scale production. For E. coli producing recombinant insulin, a 50-liter pilot system evaluates centrifugation at 6,000 g, achieving 1.8 grams per liter with 90% recovery, while identifying potential limitations in cell harvest and clarification. Similarly, pilot-scale operations reveal bottlenecks such as filter fouling during P. pastoris antibody purification, which can reduce throughput by 10% if not properly addressed.
Figure 11.11. Bioprocessing scale-up progresses from lab-scale proof of concept to pilot-scale testing and process optimization, and ultimately to industrial-scale production under GMP compliance. Each stage involves increasing volumes, more stringent process controls, and enhanced regulatory oversight to ensure that bioproducts remain consistent, safe, and of high quality. Image created with Google Gemini. Released under CC BY 4.0.
Chromatography at pilot scale is critical for validating purification strategies. For CHO cells producing monoclonal antibodies like nivolumab, 10-liter Protein A columns confirm 3 grams per liter yields with 95% purity at a cost of $5,000 per run. Modular equipment design allows adjustments such as fine-tuning flow rates, gradient elution, or column packing to optimize recovery of B. subtilis enzymes at 1.5 grams per liter. Pilot-scale studies also test integration of unit operations, including filtration, concentration, and buffer exchange, providing critical insights into material balances, process time, and robustness. Costs at pilot scale are moderate (around $1,000 per batch), but limited throughput requires careful extrapolation to predict industrial-scale performance. Computational tools, such as computational fluid dynamics (CFD), simulate mixing, aeration, and mass transfer in pilot bioreactors, ensuring that parameters like S. cerevisiae ethanol yields (50 grams per liter) scale predictably. Additionally, pilot-scale trials allow assessment of process reproducibility, process control strategies, and potential regulatory compliance challenges before committing to large-scale production.
11.8.2. Industrial-Scale Considerations
Industrial-scale processing is like operating a full production line, managing massive volumes where efficiency, yield, and cost are critical. For E. coli insulin production, 10,000-liter centrifuges at 8000 g recover 1.7 g/L, though increased shear stress can cause up to 5% product loss, necessitating optimized rotor designs. P. pastoris antibody production scales to 5000-liter systems, where large-scale chromatography columns (~$50,000) purify 2.8 g/L of antibodies such as cetuximab with 98% purity. Energy demands are substantial: centrifugation consumes 20 kWh per cubic meter, adding ~$3 per liter, while facility requirements, including cleanrooms for CHO cell antibodies, cost ~$500 per square meter annually.
For Chlorella vulgaris lipid production, 10,000-liter photobioreactors recover 1 g/L via flocculation, but light penetration limits require wider reactor designs, increasing capital expenditure by ~15%. Industrial-scale systems emphasize automation, such as continuous filtration for Aspergillus niger citric acid (20 g/L), which reduces labor costs by ~20%. Scaling up to industrial volumes demands robust, high-capacity equipment, precise process control, and strategic energy management to maintain consistent yields and product quality across all stages of biomanufacturing.
11.8.3. Key Differences
Pilot-scale operations prioritize flexibility and process optimization, allowing iterative adjustments and troubleshooting, whereas industrial-scale production emphasizes high throughput, efficiency, and cost-effectiveness. For example, pilot-scale B. subtilis enzyme processes test multiple filtration membranes, identifying a 0.2‑micron option that maximizes yield. At an industrial scale, this choice is fixed for 10,000‑liter runs to ensure consistent performance. Pilot-scale tolerates higher variability (≈10%), but industrial-scale operations require tighter control (≈2%) to meet regulatory standards.
Integrating pilot-scale data with process analytical technology (PAT), such as in-line HPLC for CHO antibodies, ensures predictable scale-up and reduces potential yield losses by ~10%. Maintaining process reproducibility and regulatory compliance is akin to ensuring every batch of cookies tastes identical while passing a health inspection. Applying these principles guarantees consistent product quality and safety, meeting stringent requirements for bioproducts ranging from pharmaceuticals to food additives.
11.8.4. Process Reproducibility
Reproducibility ensures that each batch consistently delivers the same yield, purity, and performance, much like striking the same musical note every time. In Escherichia coli insulin production, reproducible centrifugation and chromatography achieve 1.8 g/L with 99% purity across ten consecutive batches, with automated controls maintaining pH at 7.0 and flow rates at 1 L/min. Pichia pastoris antibody production attains 2.8 g/L with just 1% variability by standardizing Protein A column conditions. In Saccharomyces cerevisiae ethanol fermentations, near-infrared (NIR) spectroscopy monitors glucose levels, ensuring 50 g/L yields with 2% batch-to-batch variation. For Chlorella vulgaris lipid production, automated light control (2000 µmol/m²/s) maintains 1 g/L yields across seasonal changes, reducing variability by 15%.
Achieving reproducibility depends on robust process design, incorporating process analytical technology (PAT) and statistical process control (SPC) to minimize deviations, prevent rework, and save approximately USD 10,000 per batch. Despite these measures, challenges remaining equipment wear, and drift can increase variability by up to 5%, necessitating routine calibration and maintenance to sustain consistent bioprocess performance.
11.8.5. Regulatory Compliance
Regulatory compliance ensures bioproducts meet the stringent standards set by agencies such as the FDA or USDA, much like passing a final exam before entering the market. For CHO cell antibodies like nivolumab, compliance requires 99.9% purity, verified through HPLC and ELISA, along with thorough documentation of all process parameters. Aspergillus niger derived citric acid for food applications must meet GRAS standards, with GC-MS confirming that mycotoxin contaminants remain below 0.01 ppm. B. subtilis vaccine antigens require sterility testing, ensuring microbial contamination does not exceed 10 CFU/mL, validated through plate counts. Compliance relies on adherence to good manufacturing practices (GMP), including cleanroom protocols, which can cost $500,000 annually for facilities producing P. pastoris insulin. Failure to comply can result in batch rejection, potentially losing $100,000 per 5000-liter batch, or delaying market approval. Integrating process analytical technology (PAT) for example, in-line UV-Vis monitoring in S. cerevisiae ethanol fermentation enables real-time oversight, reducing the risk of audit failures by 20% and ensuring continuous adherence to regulatory requirements.
11.8.6. Integration and Optimization
Combining scale-up and validation strategies enhances bioprocess efficiency and reliability. For E. coli insulin, pilot-scale evaluation of centrifugation and chromatography parameters informs industrial-scale rotor and column designs, improving yield by 10% and reducing shear-induced losses. In Chlorella vulgaris lipid production, SCADA systems capture and integrate pilot-scale light and nutrient data into industrial photobioreactors, maintaining consistent yields across seasonal and operational variations. Regulatory compliance is streamlined for CHO antibodies through automated process monitoring and data logging, reducing documentation time by 15% while ensuring adherence to GMP standards. By integrating pilot-scale insights, process analytical technology (PAT), and robust monitoring systems, these approaches enable scalable, reproducible, and compliant bioprocesses, minimizing operational risk, controlling costs, and delivering consistent, high-quality bioproducts from life-saving therapeutics to renewable biofuels. Additionally, iterative feedback from pilot to industrial scale allows optimization of energy consumption, reagent usage, and equipment efficiency, further enhancing overall process sustainability.
11.9 Waste Management and By-product Valorization
Waste management and by-product valorization in bioprocessing are like turning the leftovers from a large feast into new, valuable dishes, maximizing resources while minimizing waste. Biomanufacturing processes, whether E. coli producing enzymes or Chlorella vulgaris yielding biofuels, generate both liquid effluent and solid residues that must be treated to comply with environmental regulations. Meanwhile, by-products such as spent biomass or residual media can be transformed into useful resources, including biofertilizers, animal feed, or energy substrates. Poor waste management can result in fines of up to USD 50,000 per violation or severe environmental harm, whereas smart recycling strategies can cut disposal costs by 30% and open new revenue streams. This section explores treatment approaches for wastewater and solid waste, as well as strategies to integrate by-products into a circular bioeconomy, demonstrating how sustainable bioprocessing can be both environmentally responsible and economically profitable. Through practical examples and a biotechnology perspective, we illustrate how waste management and by-product valorization are transforming biomanufacturing from therapeutic production to eco-friendly materials into a greener, more efficient, and resilient industry.
11.9.1. Liquid waste
Organic production generates both liquid effluent and solid waste, analogous to dishwater and food scraps after a meal, which must be properly treated to minimize environmental impact and comply with regulatory frameworks such as EPA standards. Effective treatment not only ensures safe disposal but also reduces operational costs and the overall environmental footprint. Effluent treatment cleans liquid waste, such as spent media or wash solutions, removing contaminants before discharge. For E. coli producing recombinant human growth hormone, fermentation broth contains approximately 10 g/L of residual glucose and ammonium salts, which are treated via activated sludge systems where Pseudomonas aeruginosa degrades organic matter, reducing chemical oxygen demand (COD) by 90% in 1000-liter batches. This process costs about $0.5 per liter and takes 24 hours, ensuring effluent COD meets discharge limits of 250 mg/L. For P. pastoris producing antibodies like trastuzumab, ultrafiltration removes proteins from 5000-liter wash solutions, recovering 95% of the water for reuse and reducing disposal costs by 20%. High-COD effluents from S. cerevisiae ethanol production are treated via anaerobic digestion, converting 50 g/L of organic waste into biogas (approximately 1 m³ per liter), offsetting energy costs by 15%. While effluent treatment is critical for regulatory compliance and environmental protection, it remains energy-intensive, adding roughly 10% to overall operating costs.
11.9.2. Solid Waste Treatment
Solid waste, including spent biomass and filtration residues, requires proper processing to reduce volume, environmental impact, and disposal costs. For B. subtilis producing polyhydroxybutyrate (PHB) for bioplastics, centrifugation generates approximately 100 kg of wet biomass per 1000-liter batch. Treatment with T. reesei degrades cell debris, reducing waste volume by 70% over two weeks. The resulting compost costs about $0.2 per kg and produces nutrient-rich fertilizer for agricultural use. In Chlorella vulgaris lipid production, 50 kg of wet algal biomass per 1000 liters is thermally dried at 80°C, yielding 10 kg of dry biomass suitable for animal feed, at a cost of $0.3 per kg. For A. niger citric acid production, filtration residues (~20 kg per 1000 liters) are incinerated at 800°C, reducing volume by 90%, though energy costs raise disposal to $1 per kg. While incineration ensures rapid volume reduction and regulatory compliance under frameworks like RCRA, it is energy-intensive. Composting and thermal drying provide sustainable, cost-effective alternatives, enabling valorization of solid residues into fertilizers or feed, thereby supporting circular bioeconomy principles and reducing the environmental footprint of bioprocessing.
11.9.3. Challenges and Optimization
Effluent and solid waste treatment in bioprocessing faces challenges such as high chemical oxygen demand (COD) and hazardous residues. For E. coli producing human growth hormones, advanced oxidation using ozone layers reduces COD by 95%, though the required equipment increases costs by $0.2 per liter. Saccharomyces cerevisiae wastewater benefits from integrating membrane bioreactors (MBRs) with anaerobic digestion, cutting treatment time by 30% and saving approximately $0.10 per liter. In Chlorella vulgaris biomass processing, combining composting with biochar production not only reduces disposal costs by 25% but also generates high-value soil amendments, contributing to circular bioeconomy principles. These optimized strategies enable compliance with environmental regulations, lower operational costs, and enhance the sustainability of bioprocess waste management, transforming potential liabilities into valuable by-products.
11.9.4. Utilization of By-products in the Circular Economy
By-product valorization transforms waste streams into valuable products, supporting a circular bioeconomy. Like turning kitchen scraps into a gourmet dish, this approach reduces waste, lowers disposal costs, and generates new revenue streams, enhancing the sustainability of biomanufacturing. Spent biomass, including microbial or algal cells, is particularly valuable. In S. cerevisiae ethanol production, 100 kilograms of yeast biomass per 1000 liters, rich in 40% protein (w/w), can be processed into animal feed supplements, generating $0.5 per kilogram in revenue. Chlorella vulgaris residual biomass from lipid extraction (50 kilograms per 1000 liters) contains 30% protein and, after drying, serves as a fertilizer additive, yielding $0.3 per kilogram. For B. subtilis PHB production, 80 kilograms of biomass per 1000 liters can be anaerobically digested to produce biogas (0.5 cubic meters per kilogram), offsetting up to 20% of energy costs. Overall, biomass valorization reduces disposal volumes by 60%, although processing steps such as drying or digestion add approximately $0.2 per kilogram in operational costs. Optimizing extraction techniques to maintain biomass integrity, as demonstrated for C. vulgaris, can increase product value by 15%. Figure 11.12 illustrates an anaerobic digestion system converting B. subtilis biomass into biogas, highlighting how 0.5 cubic meters per kilogram can offset 20% of energy consumption. This visualization underscores the critical role of by-product valorization in improving both economic and environmental sustainability in bioprocessing.
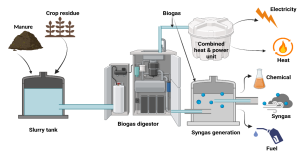
Figure 11.12. Diagram of an anaerobic digestion system, showing biogas production from biomass valorization. Mandal, A. (2025) BioRender.
11.9.5. Media and By-product Recycling
Residual media and by-products, such as sugars or organic acids, can be efficiently repurposed, like reusing a broth in a new recipe. In E. coli growth hormone production, spent media containing 5 grams per liter of glucose is recycled through ultrafiltration, recovering 90% of nutrients for reuse in fermentation, saving approximately $0.4 per liter. A. niger citric acid production generates glycerol by-products (2 grams per liter), which are purified via distillation for applications in cosmetics, yielding $1 per liter in revenue. In P. pastoris antibody production, ammonium salts present in wash solutions are recovered using ion exchange and repurposed as fertilizer, generating $0.2 per liter. Recycling strategies reduce waste treatment costs by 25%, though purification steps add about $0.1 per liter. Integrating processes, such as coupling ultrafiltration with fermentation for E. coli, further enhances nutrient recovery by 10%, improving both sustainability and cost-efficiency in biomanufacturing.
11.9.6. Circular Economy Applications
By-product valorization enables a circular economy by transforming waste streams into valuable products, creating closed-loop systems. In Chlorella vulgaris lipid production, spent biomass is converted into biochar, enhancing soil carbon sequestration and generating $0.5 per kilogram. S. cerevisiae yeast extracts are repurposed as flavor enhancers in food products, adding $0.3 per kilogram in value. By-products from B. subtilis PHB production are processed into bioplastic additives, reducing raw material costs by 15%. These strategies support sustainability goals, cutting waste disposal by 50% and creating revenue streams worth $10,000 per 10,000-liter batch. Challenges include fluctuating market demand and high processing costs, which can erode potential profits if not carefully managed. Applying process analytical technologies (PAT), such as near-infrared (NIR) spectroscopy for monitoring A. niger glycerol content, enhances quality assurance and can improve marketability by up to 20%, ensuring consistent and high-value by-product utilization.
11.9.7. Integration and Optimization
Integrating waste management and by-product recycling enhances overall efficiency and sustainability in bioprocessing. For Escherichia coli producing growth hormone, combining wastewater ultrafiltration with biomass composting reduces treatment costs by 30% while recovering valuable nutrients for reuse. In Chlorella vulgaris cultivation, coupling biochar production with lipid extraction generates a dual revenue stream of biofuels and soil amendments, saving approximately USD 0.20 per liter of culture. Automated SCADA systems applied to S. cerevisiae biogas production optimize fermentation parameters, monitor real-time process variables, and improve yield by 15%. Additional strategies, such as integrating membrane bioreactors with anaerobic digestion or valorizing residual sugars and proteins, further enhance resource efficiency and reduce environmental impact. These integrated approaches minimize waste, enable recovery of valuable by-products, and ensure that biological processing adheres to circular economy principles, ultimately supporting sustainable production while maintaining high-quality, economically viable bioproducts.
11.10 Conclusion
Downstream processing serves as the critical bridge between bioreactor output and market-ready bioproducts. It is not merely an end-stage cleanup but a carefully orchestrated sequence of operations that determines commercial viability, regulatory compliance, and environmental sustainability. Recovery and purification dominate production costs, often representing 50 80% of total expenses. For instance, CHO cell antibody purification relies on expensive Protein A chromatography, whereas commodity products such as citric acid from A. niger employ leaner operations like precipitation. Balancing purity with yield is a constant trade-off, as each purification step improves quality but can incur 5 10% product loss. Integration with upstream decisions is equally important. Engineering E. coli to secrete proteins reduces cell lysis costs, while optimized C. vulgaris cultures lower downstream burdens in lipid extraction. Continuous processes, such as perfusion bioreactors coupled with chromatography, illustrate the drive for efficiency and scalability, though they require significant capital investment. Technological innovation strengthens this framework: tools like HPLC, ELISA, and NIR spectroscopy ensure purity and regulatory compliance, while process analytical technology (PAT) enables real-time monitoring, reducing variability and minimizing rework. Reproducibility and documentation are essential, as evidenced in insulin and monoclonal antibody production, where consistent quality and adherence to FDA or GMP standards safeguard both patient safety and process reliability.
Sustainability is increasingly central to downstream processing. Effluent treatment, biomass composting, and by-product valorization transform waste into valuable resources. For example, S. cerevisiae wastewater treated by membrane bioreactors and Chlorella biomass converted into soil amendments demonstrate circular economy principles, cutting disposal costs and generating revenue, while poor waste management risks fines or environmental damage. Downstream processing is thus both a science and an art, requiring mastery of separation technologies, process economics, and regulatory frameworks alongside creative integration. From purifying delicate therapeutic proteins to valorizing industrial by-products, each decision shapes the success of biomanufacturing at scale. Advances in continuous systems, microfluidic purification, and digital controls promise to further streamline operations, reduce costs, and enhance sustainability. Ultimately, downstream processing is the decisive stage where biotechnology translates laboratory innovation into consistent, safe, affordable, and sustainable products with global impact.
Resources
- OpenStax Biology 2e: https://openstax.org/details/books/biology-2e
- Wikimedia Commons Microbial Biotechnology Images: https://commons.wikimedia.org/w/index.php?search=microbial+biotechnology
Recommended Reading Materials
- Aguilar, O., & Rito-Palomares, M. (2008). Aqueous two-phase systems for protein separation. Journal of Chromatography B, 876(1), 15 22.
- Carta, G., & Jungbauer, A. (2010). Protein Chromatography: Process Development and Scale-Up. Wiley-VCH.
- Flickinger, M. C. (2010). Encyclopedia of Industrial Biotechnology. Wiley.
- Glassey, J., et al. (2011). Process Analytical Technology in Bioprocessing. Advances in Biochemical Engineering/Biotechnology.
- Gottschalk, U. (2009). Process Scale Purification of Antibodies. Wiley.
- Harrison, R. G., et al. (2015). Bioseparations Science and Engineering. Oxford University Press.
- Janson, J. C. (2011). Protein Purification: Principles, High Resolution Methods, and Applications. Wiley.
- Kosseva, M. R. (2013). Food Industry Wastes: Assessment and Recuperation of Commodities. Academic Press.
- Ravindran, V., & Jaiswal, A. K. (2016). By-Product Valorization in Bioprocessing. Bioresource Technology.
- Walsh, G. (2018). Bioprocess Engineering. Wiley.
- Wang, L. K., et al. (2009). Environmental Biotechnology. Humana Press.

