5 Chapter 5. Regulation of Gene Expression
Albert B. Flavier
Chapter Outline
5.2 Regulation of the lac operon
5.3 Regulation of the ara operon
5.4 Regulation of the trp operon and attenuation
Learning objectives
By the end of this chapter, you will be able to:
- Contrast negative and positive regulation
- Describe effectors and their effect on gene regulation
- Describe the positive and negative inducible regulation of the lac operon
- Explain catabolite repression
- Describe the negative and positive inducible regulation of the ara operon
- Describe the negative regulation of the trp operon by TrpR
- Explain gene regulation by attenuation
5.1 What is gene regulation?
For the most part, there are identical sets of genes in every cell in an organism’s body. Yet, if genotype determines the phenotype, how does a nose end up being a nose, an eye an eye, and the brain anatomically and functionally differently from muscles? The explanation is that there are different sets of genes getting transcribed and translated into proteins with different activities and functions in those organs. However, it is not only at this present time while you’re studying the course material when gene expression patterns differ in those organs. At different times during the course of one’s development from a single–celled embryo to a college student, the right sets of genes would have to be turned on and off at precise times, or you would end up looking at a very different image in the mirror. Without proper gene regulation in place, if there is dysregulation, there would be chaos. There would be uncontrolled cell division leading to cancer or some other disease. There would be no development of leaves, roots, flowers, or seeds in a plant, and in many organisms, including microbes, it could be a matter of survival.
Gene regulation is simply the turning on and off of gene expression. Gene regulation is designed to increase or decrease RNA or protein levels depending on the needs of the cell. A cell expresses only a fraction of its genome at any given time. Regulation of gene expression can occur at any stage in the expression of the gene, i.e., transcriptional, post-transcriptional, translational, and/or and post-translational. However, regulation at transcriptional level would be most efficient in saving cellular energy and resources. Why? It is very energy demanding to synthesize amino acids, proteins, ribonucleotides and RNA. If biosynthetic activity can be stopped before transcription even starts then that’s more energetically wise. When mRNA is synthesized, not only does the cell use up resources for the synthesis of the ribonucleotides as building blocks, RNA polymerase activity also uses up energy. Post-translational regulation, by regulating stability or activity of proteins is most resource consuming since mRNA and proteins have already been synthesized and the building blocks already used up. In addition, if protein levels are controlled post-translationally, enzymes and energy will be involved in the degradation of proteins and recycling of amino acids. This chapter will focus on regulating gene expression at the transcription level but would also touch on other forms of gene expression regulation.
In organisms, genes can be classified as regulatory and structural depending on the role of the proteins they code for (Fig. 5.1). Regulatory genes code for proteins that control the transcription or translation of other genes, while structural genes code for proteins that have structural, transport, or enzymatic functions. Regulatory proteins include transcriptional activators and repressors. Activators bind to control regions of genes which are referred to as enhancers, while repressors bind to sequences which are termed operators.
In prokaryotes (bacteria and archaea), many genes that code for proteins that have related functions are organized into operons (Fig. 5.1). An operon is a single transcriptional unit where two or more genes are on the same DNA strand and transcribed together under the control of a common regulatory region. The mRNA transcribed from the operon is a polycistronic mRNA which encodes two or more proteins that get translated separately. An operon’s regulatory region includes the promoter and can include an operator and/or enhancer sequences.
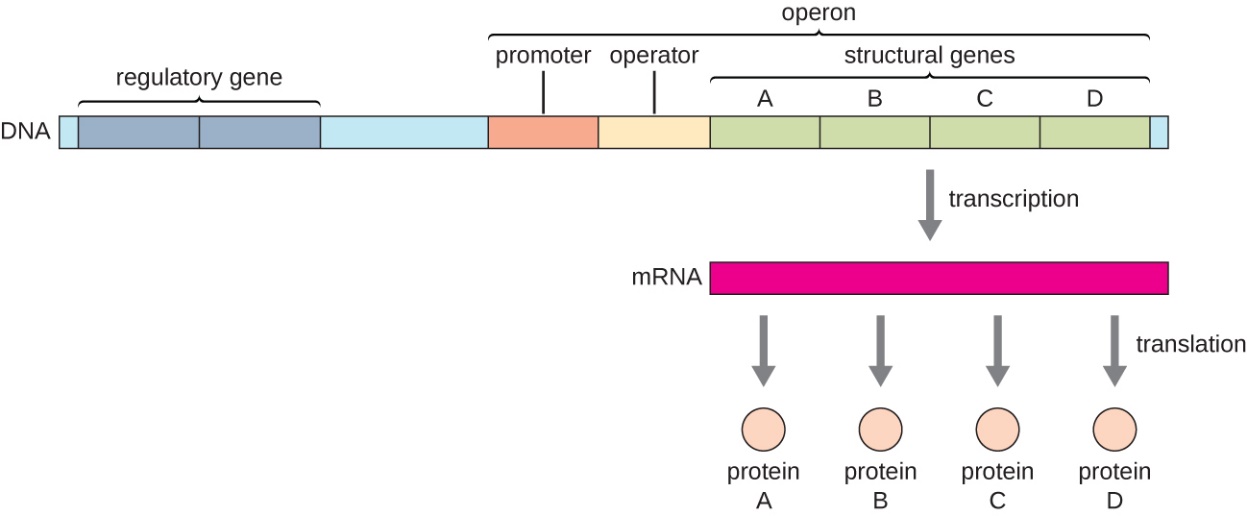
Figure 5.1. In prokaryotes, operons consist of two or more structural genes of related functions which are often transcribed together under the control of a single promoter. The operon’s regulatory region includes both the promoter and the operator. The transcribed polycistronic mRNA will get translated into two or more protein products. CC-SA-4.0 Parker et al.
Gene regulation can be classified as negative or positive regulation.
- Negative regulation – a transcriptional repressor binds to the operator, and the structural genes do not get transcribed. A null mutation which leads to inactivation of the repressor protein or to a lack of its expression would result in constitutive expression of the repressed gene/s. Repressors can block transcription by:
- Directly interfering with the RNA polymerase binding to the promoter
- Prevent melting of the DNA at the promoter
- Latch on to the RNA polymerase and prevent its escape from the promoter
- Away from the promoter, repressors can bend the DNA, such that contact of RNA polymerase with the promoter is no longer optimal
- Positive regulation – a transcriptional activator binds to an enhancer region to increase transcription levels. Transcription can be increased a thousand-fold in the presence of an activator. A null mutation which leads to inactivation of the activator protein or to a lack of its expression would result to reduced, or absence of expression of the regulated gene/s. Activators can increase transcription by:
- Guiding the RNA polymerase to the promoter.
- Helps anchor the RNA polymerase to the promoter sequences
- Restructure the promoter to orient the promoter sequences to favor contact with the RNA polymerase.
In bacteria, many genes whose products have enzymatic activities can be divided into 2 groups:
- Biosynthetic enzymes – Synthesize organic compounds and can include formation or assembly of macromolecules (protein, polynucleotide, membranes, organelles, etc.) or a building block for macromolecules (amino acids, nucleotides, fatty acids) from simpler chemicals or building blocks
- Catabolic enzymes – Are involved in degrading or breaking down cellular material including ribosomes, nucleic acids, proteins, and complex organic compounds into simpler molecules as a source of energy or building blocks for the synthesis of new macromolecules. Catabolic enzymes are also important in detoxification of an antibiotic or other harmful compounds like organophosphates, or a harmful cell-produced metabolite.
In prokaryotes, there are operons whose gene products are required consistently by the cell. Such operons are described as constitutive, meaning they are transcribed and translated continuously to provide the cell with constant intermediate levels of the protein products. Such genes encode enzymes involved in housekeeping functions required for cellular maintenance, including DNA replication, repair, transcription and translation, as well as enzymes involved in core metabolism, like respiration. In contrast, there are other prokaryotic operons that are expressed only when needed during certain conditions and are regulated by repressors, activators, and effector molecules.
Effector molecules are compounds that binds to transcriptional regulators and either increase or decrease binding of the regulatory proteins to target DNA sequences. Thus, operons can be classified as:
- Negative inducible operons – a regulatory repressor protein is normally bound to the operator, which prevents transcription and certain molecules (inducers) can bind to the repressor and change its conformation (shape) so that it is unable to bind to the operator, thus allowing for expression of the operon. Example: E. coli LacI repression of lac operon and induction in the presence of lactose.
- Negative repressible operons – transcription of the operon normally takes place. That’s because the repressor proteins are unable to bind to the operator until they are associated with a molecule which is corepressor that causes a conformational change to the repressor which then increases repressor binding to operator. E. coli trp operon repression by TrpR in the presence of tryptophan as co-repressor.
- Positive inducible operons – By itself, the activator proteins are normally unable to bind to the gene’s enhancer region. In the presence of inducers, the activator changes conformation and is able to bind to enhancer regions and drive transcription. Example: E. coli lac operon activation by CAP in the presence of cAMP as inducer.
- Positive repressible operons – Activator proteins are normally bound to the enhancer region. An effector molecule, an inhibitor, binds to the activator and weakens its binding to enhancer sequences. Example: E. coli fab operon activation by FadR which falls off in the presence of certain fatty acids as inhibitors.
Effector molecules work by changing the structure of repressor or activator proteins, and either weaken or enhance their association with operators or enhancers, respectively.
Inducers – Results in an operon being transcribed. Inducers work by inactivating a repressor or activating binding of an activator.
Co-repressor – Results in the operon being repressed (no transcription) by enhancing the binding of a repressor to operator sequences.
Inhibitors – Weakens the binding of activators to enhancers leading to operons getting repressed.
Some regulatory proteins can act as repressors or activators depending on presence or absence of effectors or the type of effector molecule present. The binding sites on DNA can be different or the same in the presence or absence of the effector
Sequences recognized and bound to by regulatory proteins can change also in the presence of effectors. The affinity for certain sequences increases, while affinity for a another set of sequences decreases.
The lac operon (Fig. 5.2) is an example of a negative inducible operon. The lac operon encodes three structural genes, lacZYA, where lacZ codes for b-galactosidase (LacZ) which breaks down the disaccharide lactose into the simple sugars, glucose and galactose (Fig. 5.3), LacY which is a permease which facilitates lactose uptake from the environment, and LacY (an acetylase whose role is uncertain). A lac repressor, LacI, binds to the operator region (lacO) downstream of the lac operon promoter, plac, and physically preventing RNA polymerase from transcribing lacZYA (Fig. 5.2). However, when lactose is present, lactose is converted to the intermediate, allolactose, whereby allolactose serves as an inducer, binding to LacI and changing its shape so that it is no longer able to bind to lacO. Dissociation of LacI from lacO in the presence of lactose allows RNA polymerase to move through the operator region and begin transcription of the lac structural genes. Therefore, for the lac operon to be expressed, lactose must be present. This makes sense because it would be wasteful for the cell to synthesize the enzymes to process lactose if lactose is not available.
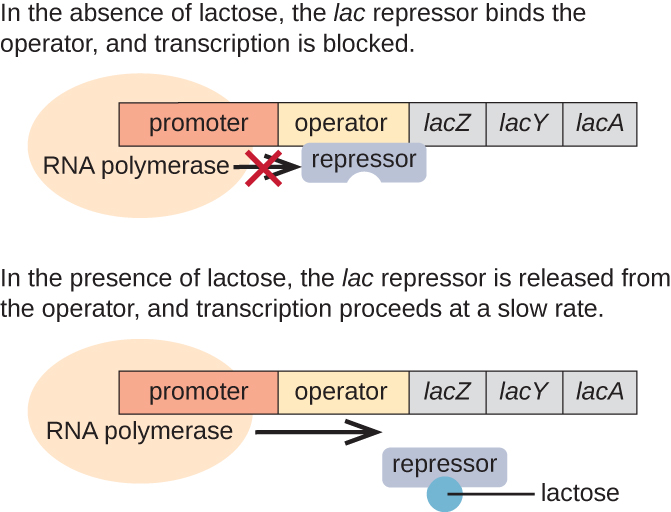
Figure 5.2 The three structural genes that are needed to degrade lactose in E. coli are located next to each other in the lac operon. The repressor protein, LacI, binds to the operator, physically blocking the RNA polymerase from transcribing the lac structural genes. When lactose is available, a lactose derivative binds to LacI, causing it to change shape and preventing if from binding to the operator sequence, and the downstream genes are transcribed. CC-SA-4.0 Parker et al.
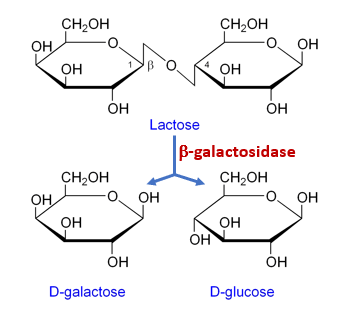
Figure 5.3 The enzyme, b-galactosidase, (LacZ) hydrolyzes the disaccharide, lactose, into the monosaccharides galactose and glucose. Released to public domain by Flavier.
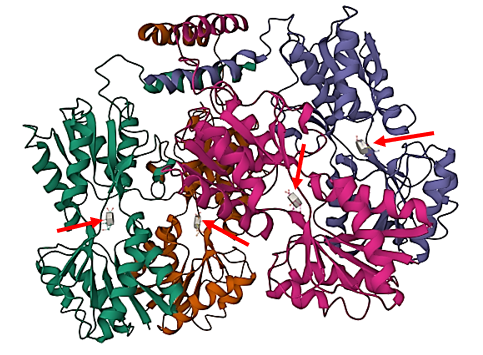
Figure 5.4 The 3-dimensional structure of LacI and the inducer, IPTG. The transcriptional repressor, LacI, is a tetramer of identical subunits (homotetramer). Each subunit contains a helix-turn-helix (HTH) motif capable of binding to DNA. The operator site where LacI binds is a DNA sequence with inverted repeat symmetry. The two DNA half-sites of the operator together bind to two of the subunits of LacI. In the presence of the gratuitous inducer, IPTG (gray molecules near tip of red arrows), LacI changes shape and weakens its hold on the operator regions. Image is public domain PDB accession 1LBH by Lewis et al.
The lac operon is also a positively regulated, inducible operon. The catabolite activator protein, CAP, binds to an enhancer region (CAP-binding sequence, CBS) upstream of the promoter and increases transcription, but only in the presence of cyclic AMP (cAMP) as inducer (Fig 5.5). Thus, CAP is also known as cAMP receptor protein (CRP). Biosynthesis of cAMP from ATP by the membrane-localized enzyme, adenylate cyclase, increases when there are low levels of glucose, and cAMP decreases in the presence of high levels of glucose. Thus, at low glucose levels and in the presence of lactose, there is increased transcription of the lac operon which enables utilization of lactose as alternative carbon source. At high glucose levels, cAMP levels decreases and the lac operon is not highly expressed. To conserve cellular resources, it makes sense to shut off expression of the lac operon when its products are not needed.
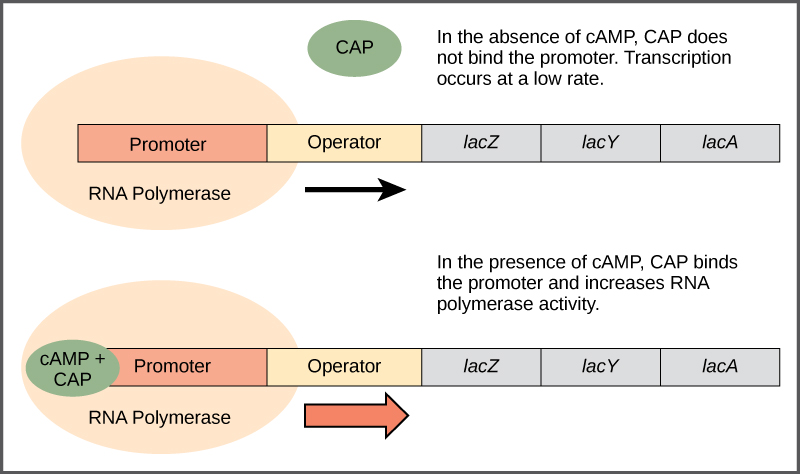
Figure 5.5 When glucose levels fall, E. coli may use other sugars for fuel but must transcribe new genes to do so. As glucose supplies become limited, cAMP levels increase. cAMP binds to the CAP protein, a transcriptional activator that binds to an enhancer region upstream of the genes required to use alternative carbon sources. CC-SA-4.0 Parker et al.
Bacteria typically have the ability to nutritional shift and use a variety of substrates as carbon sources, in addition to glucose and lactose. Since glucose is usually energetically preferable to other substrates, bacteria have mechanisms to ensure that alternative substrates are only used when glucose has been depleted. Additionally, bacteria have mechanisms to ensure that the genes encoding enzymes for using alternative substrates are expressed only when the alternative substrate is available. In the 1940s, Jacques Monod demonstrated E. coli’s preference for certain substrates over others through his studies of E. coli grown in the presence of two different substrates. Such studies generated diauxic growth curves (Fig 5.6) where the preferred substrate glucose is used first and enables E. coli to grow quickly; during that first phase of rapid growth, the enzymes for lactose metabolism are absent. However, once glucose levels are depleted, growth rates slow down, and expression of the enzymes (LacZYA) needed for the metabolism of the second substrate, lactose, is induced. Notice how the growth rate in lactose is slower, as indicated by the lower steepness of the growth curve.
The lac operon also plays a role in this switch from using glucose to using lactose. When glucose is scarce, the accumulating cAMP caused by increased adenylyl cyclase activity binds to CAP. In the regulatory regions of the lac operons, the CAP binding site is located upstream of the RNA polymerase binding site in the promoter. Binding of the CAP-cAMP complex to this site increases the binding ability of RNA polymerase to the promoter region to initiate the transcription of the structural genes. Thus, in the case of the lac operon, for transcription to occur, lactose must be present (to favor LacI dissociation) and glucose levels must be depleted (allowing binding of CAP-cAMP). When glucose levels are high, there is catabolite repression of operons encoding enzymes for the metabolism of alternative substrates. Because of low cAMP levels under these conditions, there is an insufficient amount of the CAP-cAMP complex to enhance transcription of these operons. See Table 5.1 for a summary of the regulation of the lac operon under different conditions.
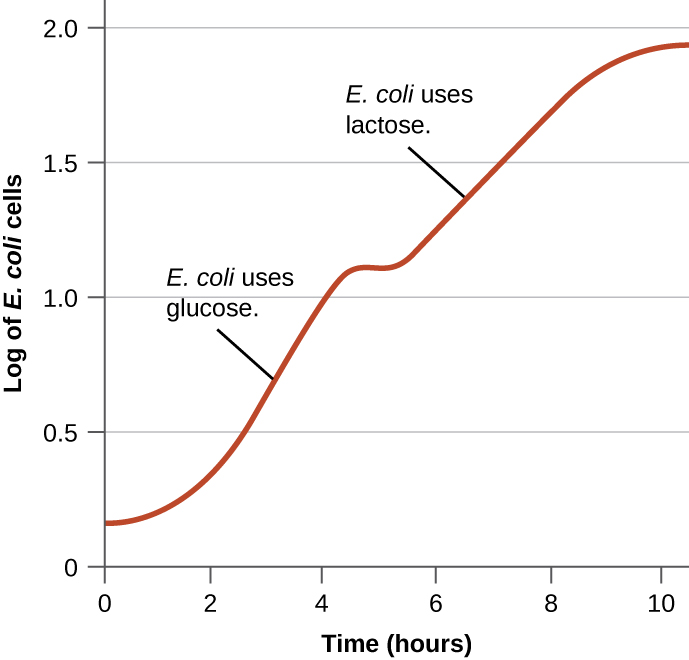
Figure 5.6 Diauxic growth curve observed in the presence of glucose and lactose as carbon sources. E. coli cell density increases exponentially while preferentially using glucose, then growth flattens as glucose is used up, before resuming exponentially while using lactose. CC-SA-4.0 Parker et al.
Table 5.1. Effects of different conditions on transcription of the lac operon. CC-SA-4.0 Parker et al.
| Glucose | CAP binding | Lactose | Repressor binding | Transcription |
| + | + | No | ||
| + | + | Some | ||
| + | + | No | ||
| + | + | Yes | ||
5.3 Regulation of the araBAD operon
Learning Objectives
By the end of this section, you will be able to:
- Describe the negative and positive inducible regulation of the ara operon
Arabinose is another alternative carbon source for E. coli growth. Arabinose is a five-carbon sugar converted by the enzymes, AraB, AraA and AraD to xylulose-5-phosphate which feeds into the pentose-phosphate pathway (PPP). PPP generates NADPH and pentoses (5-carbon sugars) as well as ribose 5-phosphate (Fig. 5.8), a precursor for the synthesis of nucleotides. The structural genes for AraB, AraA, and AraD are arranged in an operon (Fig. 5.7). The same gene locus also contains a regulatory gene, araC, whose product AraC is a repressor of the araBAD operon as well as of its own expression. In the control region, there are two operator sites, araO1 and araO2, and one inducer site, araI ( araI1 and araI2). All of these sites can be bound by AraC. AraC binds to araO2 and araI1 (Fig. 5.9), The resulting AraC dimer causes looping of the DNA strand which blocks the binding of RNA polymerase to the promoter, paraBAD, thus repressing transcription.
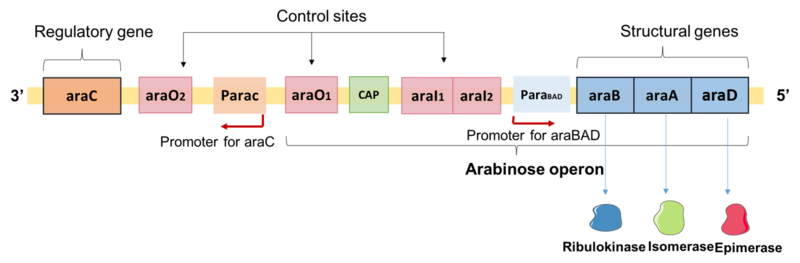
Figure 5.7. Gene organization of the arabinose catabolism locus. Shown is the gene that codes for AraC, the control sites for binding of AraC, and the araBAD operon and the encoded enzyme products of the three genes. CC-BY-SA-4.0 Yiktingg1.
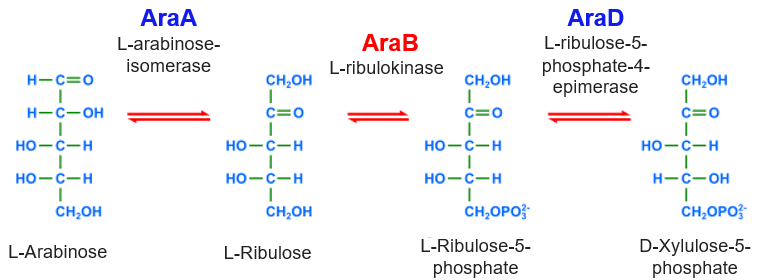
Figure 5.8 Intracellular arabinose is converted to xylulose-5-phosphate by the AraBAD enzymes. Modified from Kaidor (public domain).
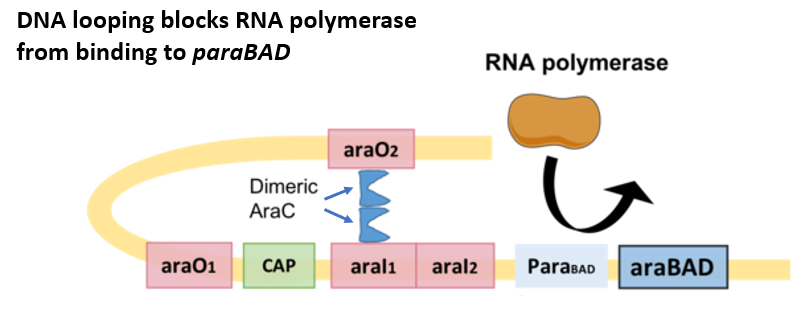
Figure 5.9. Negative regulation of the araBAD operon by AraC. In the absence of arabinose, the AraC dimer binds to araO2 and araI1 and promotes looping of the DNA. The loop interferes with successful binding of RNA polymerase to paraBAD, thus leading to low araBAD expression. CC-BY-SA-4.0 Yiktingg1.
In the presence of arabinose as inducer, AraC changes shape and switches from being a transcriptional repressor to a transcriptional activator. AraC-arabinose loses affinity for araO2 and instead binds to araI1 and araI2 (Fig. 5.10) which resolves the looped structure and increases RNA polymerase access to the paraBAD. The araBAD operon is also subject to catabolite repression. At low glucose levels, CAP-cAMP is able to bind to an enhancer region, and together with AraC and RNA polymerase drives high level transcription of araBAD. At high glucose and low cAMP levels, araBAD expression is decreased even in the presence of arabinose.
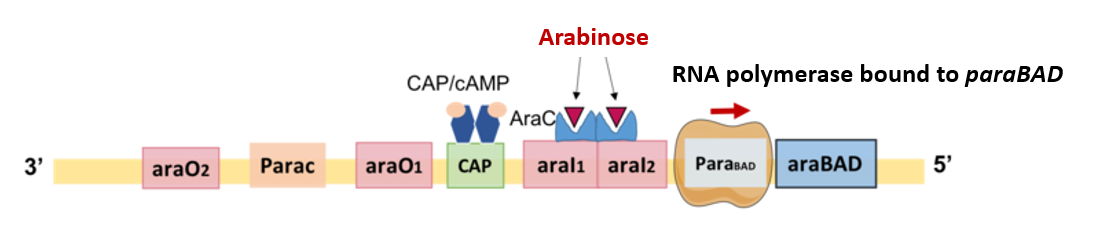
Figure 5.10. Positive regulation of the araBAD operon by AraC and CAP with arabinose and cAMP, respectively, as inducers. Arabinose when present, binds to the AraC dimer. The resulting change in the shape of AraC promotes its binding to araI1 and araI2 which then allows binding of RNA polymerase to the paraBAD. In the presence of cAMP-activated CAP, transcription of araBAD is increased. CC-BY-SA-4.0 Yiktingg1.
5.4 Regulation of the trp operon
Learning Objectives
By the end of this section, you will be able to:
- Describe the negative regulation of the trp operon by TrpR
- Explain gene regulation by attenuation
While the lac and ara operon are both catabolic operons, the trp operon is involved in the biosynthesis of tryptophan. E. coli can synthesize tryptophan using biosynthetic enzymes that are encoded by five structural genes located next to each other in the trp operon (Fig 5.11). When intracellular tryptophan is low, the operon is transcribed, the biosynthetic enzymes are expressed, and tryptophan is synthesized. However, if intracellular tryptophan levels are high, the trp operon is turned off. Transcription does not occur, and tryptophan synthesis stops.
When tryptophan is not present in the cell, the repressor, TrpR, by itself does not bind to the operator; therefore, the operon is active and tryptophan is synthesized. However, when tryptophan accumulates in the cell, two tryptophan molecules bind to TrpR, which changes its shape, favoring its binding to the trp operator. Binding of the active form of TrpR to the operator blocks RNA polymerase from transcribing the structural genes, stopping expression of the operon. Thus, the product of the biosynthetic pathway controlled by the operon regulates the expression of the operon.
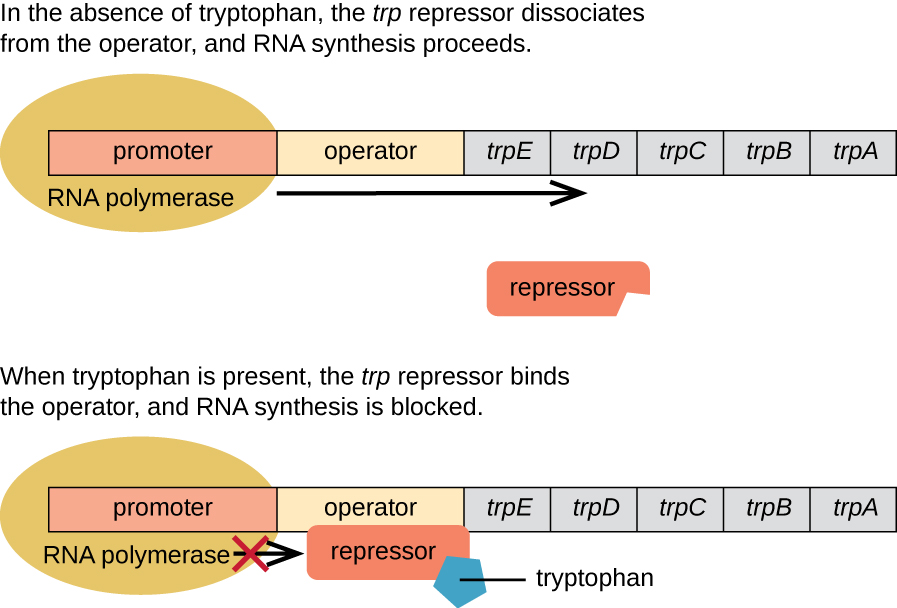
Figure 5.11 The five structural genes needed to synthesize tryptophan in E. coli are located next to each other in the trp operon. When tryptophan is absent, the repressor protein does not bind to the operator, and the genes are transcribed leading to biosynthesis of tryptophan. When tryptophan is plentiful, tryptophan binds to the repressor protein, TrpR, and enhances TrpR’s binding to the operator sequence. Thus TrpR-tryptophan physically blocks the RNA polymerase from binding to the promoter and transcribing the tryptophan biosynthesis genes. CC-SA-4.0 Parker et al.
Additional Methods of Regulation in Bacteria: Attenuation and Riboswitches
Although most gene expression is regulated at the level of transcription initiation in prokaryotes, there are also mechanisms to control both the completion of transcription as well as translation concurrently. Since their discovery, these mechanisms have been shown to control the completion of transcription and translation of many prokaryotic operons. One such regulatory system is attenuation, whereby secondary stem-loop structures formed within the 5’ end of an mRNA being transcribed determine if transcription to complete the synthesis of this mRNA will occur and if this mRNA will be used for translation.
Beyond the transcriptional repression mechanism already discussed, attenuation also controls expression of the trp operon in E. coli (Fig. 5.12). The trp operon regulatory region contains a leader sequence called trpL between the operator and the first structural gene. trpL has four stretches of RNA sequences that can base pair with each other in different combinations. When a terminator stem-loop forms between regions 3 and 4, transcription terminates, releasing RNA polymerase from the mRNA. When levels of tryptophan are low, there is deficiency in the cellular levels of trp-tRNA. The ribosome stalls when it encounters the two adjacent trp codons on the trpL mRNA. Ribosome stalling leads to the formation of an antiterminator stem-loop between regions 2 and 3 which prevents the formation of the terminator stem-loop between regions 3 and 4, and so RNA polymerase can continue on to transcribe the structural genes. TrpR repression reduces trp operon transcription by 70-fold, while attenuation reduces by 10-fold for a total of 700-fold reduction in transcription.
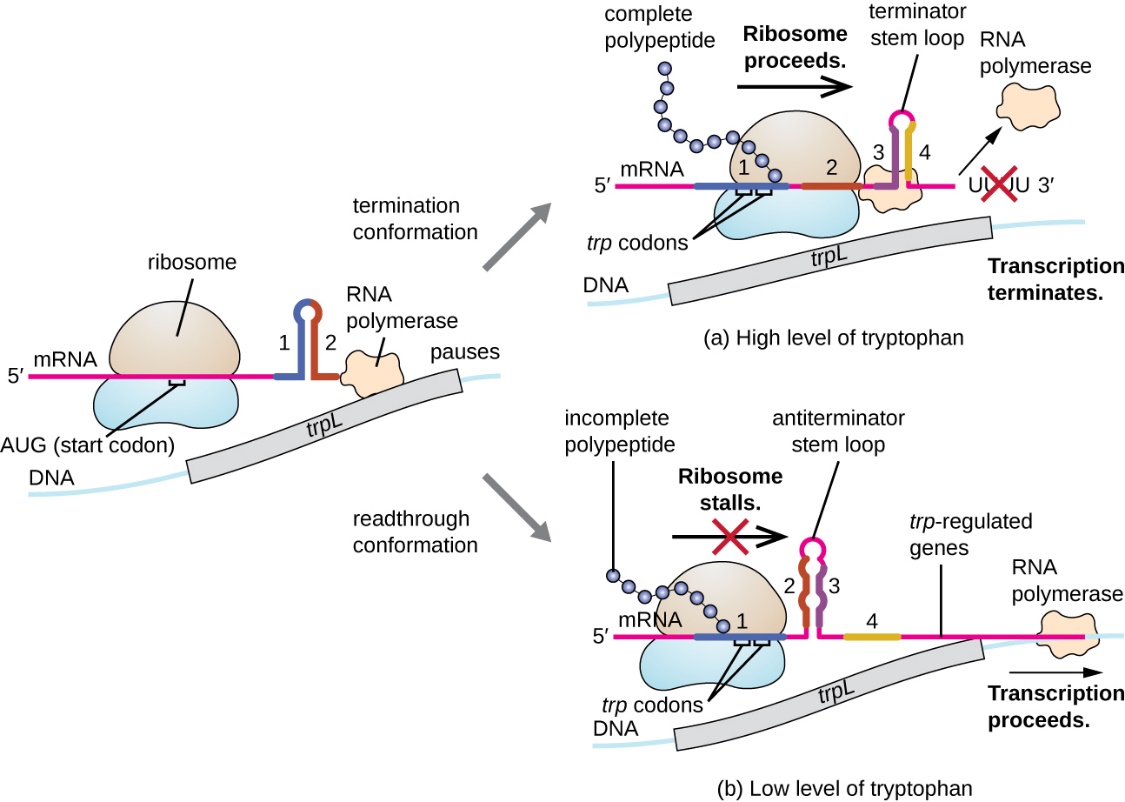
Figure 5.12 When tryptophan is plentiful, translation of the short leader peptide encoded by trpL proceeds, the terminator loop between regions 3 and 4 forms, and transcription terminates. When tryptophan levels are depleted, translation of the short leader peptide stalls at region 1, allowing regions 2 and 3 to form an antiterminator loop, and RNA polymerase continues to transcribe the structural genes of the trp operon. CC-SA-4.0 Parker et al.
A related mechanism of concurrent regulation of transcription and translation in prokaryotes is the use of a riboswitch, a small region of noncoding RNA found within the 5’ end of some prokaryotic mRNA (Fig. 5.13). In attenuation translation initiation is required, but not with riboswitch. A riboswitch may bind to a small intracellular molecule to stabilize certain secondary structures in the mRNA. The binding of the small molecule determines which stem-loop structure forms, thus influencing the completion of mRNA and protein synthesis.
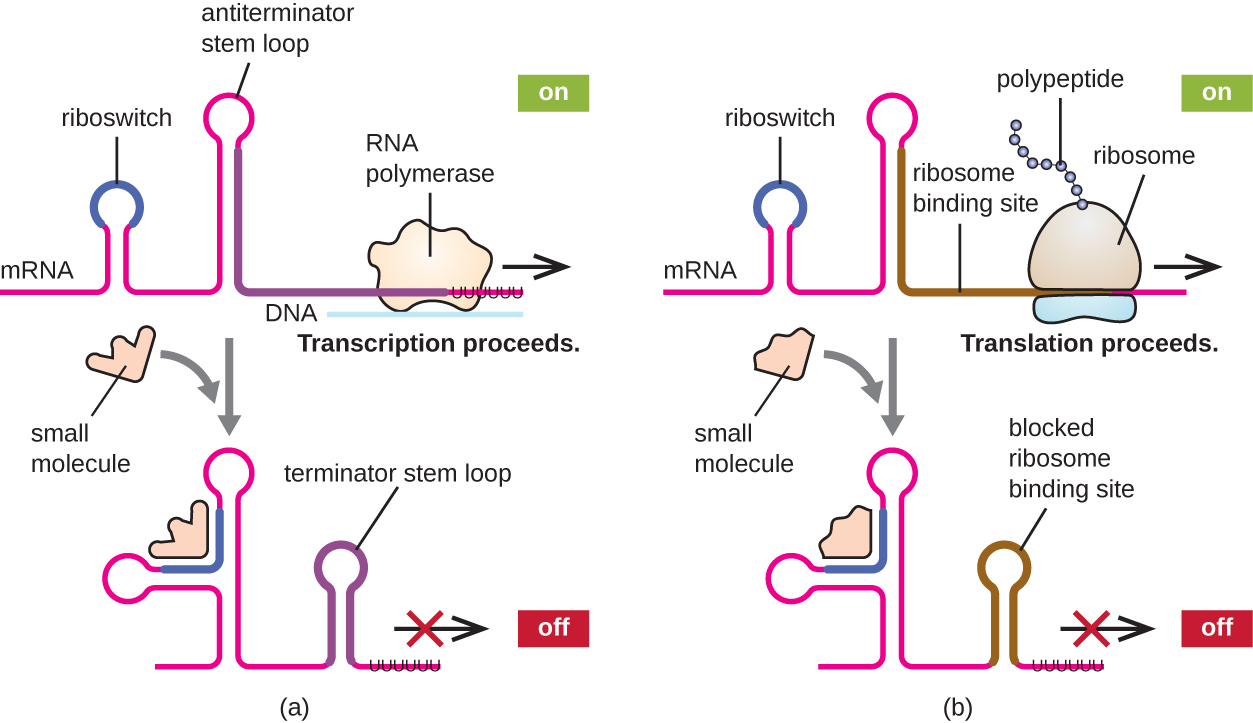
Figure 5.13 Riboswitches found within prokaryotic mRNA molecules can bind to small intracellular molecules, stabilizing certain RNA structures, influencing either the completion of the synthesis of the mRNA molecule (left) or the protein made using that mRNA (right). CC-SA-4.0 Parker et al.
Post-translational regulation of tryptophan biosynthesis
Tryptophan biosynthesis is also regulated at the post-translational level after the structural proteins have been translated. The end product of the biosynthetic pathway, tryptophan, when in excess, binds to the first (rate limiting) enzyme, anthranilate synthetase, which converts chorismate to anthranilic acid (Fig. 5.14). Anthranilate synthetase subunits are encoded by trpE and trpD. This negative feedback inhibition of a key enzyme is a common regulatory mechanism in many biosynthetic pathways in different microbes. overproduction of amino acids and other industrial products. Mutant strains with enzymes that fail to bind to the inhibitory metabolites are desired for dysregulated overproduction of amino acids and other industrial products. To reduce feedback inhibition, certain mutants are defective also in the uptake of the inhibitory amino acids from outside the cells.
.
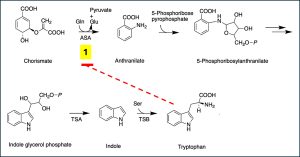 Figure 5.14. Post-translational regulation of the trp operon by negative feedback inhibition. The end product of the biosynthetic pathway, tryptophan, binds to the first (rate limiting) enzyme, anthranilate synthetase, (ASA) and inhibits the activity of the enzyme. Modified from public domain by Hyk021
Figure 5.14. Post-translational regulation of the trp operon by negative feedback inhibition. The end product of the biosynthetic pathway, tryptophan, binds to the first (rate limiting) enzyme, anthranilate synthetase, (ASA) and inhibits the activity of the enzyme. Modified from public domain by Hyk021
Global regulation
Global regulation involving alternate sigma factors
Since the σ subunit of bacterial 6-subunit RNA polymerase holoenzyme confers specificity as to which promoters should be transcribed, altering the σ factor used is another way for bacteria to quickly and globally change what regulons are transcribed at a given time. The σ factor recognizes sequences within a bacterial promoter, so different σ factors will each recognize slightly different promoter sequences. In this way, when the cell senses specific environmental conditions, it may respond by changing which σ factor it expresses, degrading the old one and producing a new one to transcribe the operons encoding genes whose products will be useful under the new environmental condition. Table 5.2 lists different sigma factors involved in transcription in response to different growth conditions. In the sporulating Gram-positive bacteria of the genera Bacillus and Clostridium (which include many pathogens), a group of σ factors controls the expression of the many genes needed for sporulation in response to sporulation-stimulating signals.
| Sigma factor | Conditions |
|
RpoD |
Housekeeping |
|
RpoE |
Extracytoplasmic stress |
|
RpoN |
Nitrogen starvation |
|
RpoH |
Heat shock |
|
RpoS |
Starvation and stress |
Table 5.2. Alternate sigma factors and the conditions which favor their association with the core polymerase to form the RNA polymerase holoenzyme.
End-of-Chapter Questions:

