7 Chapter 7. Bacteriophages
Albert B. Flavier
Chapter Outline
7.3 Bacteria defenses against bacteriophages
7.4 Bacteriophage use in medicine
Learning Objectives
By the end of this chapter, you will be able to:
- Describe bacteriophages
- Contrast the lytic and lysogenic life cycles of bacteriophages
- Describe the bacteriophage infection process
- Compare and contrast generalized and specialized transduction
- Describe bacteria defenses against bacteriophages
- Describe the use of bacteriophage in medicine
7.1 What are bacteriophages?
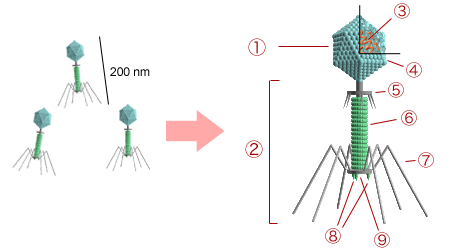
Figure 7.1. Structure of bacteriophage T4. 1 – head; 2 – tail; 3 – nucleic acid; 4 – capsid; 5 – collar; 6 – sheath; 7 – tail fiber; 8 – spikes; 9 – baseplate. CC-SA-3.0 from Y_tambe.
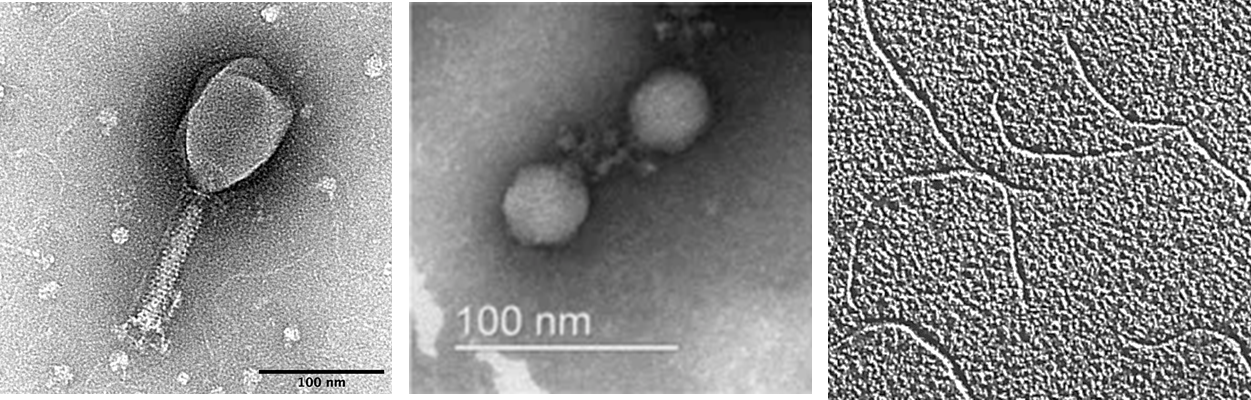
Figure 7.2. Electron micrographs of a icosahedral bacteriophage with a long tail (left) and a short tail (middle), and a filamentous bacteriophage (right). CC-SA-4.0 by SnaxMikn (left), Cha et al (middle) and Androidpar (right).
Bacteriophages (phages) are viruses that infect bacteria. Phages are the most abundant “organism” in the biosphere and in nature. It is estimated that there are around 10-100 phages for every bacterial cell. In the human body, it is estimated that there are 1015 phages – greatly exceeding the 30-100 trillion human cells. Many bacteriophages have icosahedral heads which can have long or short tails, while a few have no tails and some are filamentous (Fig 7.1 and 7.2). The genome is either RNA or DNA, with the smallest genome less than 3000 bases and the longest exceeding 300,000 bases. Like all other viruses, phages depend on their host cells for reproduction and metabolic processes. Phages do not encode all of the enzymes necessary for their replication and instead will commandeer the host’s cellular machinery to produce even more viral particles.
Watch: Virus vs. Superbug
What is an advantage of bacteriophages over antibiotics in the treatment of Superbugs?
What is an advantage of antibiotics over bacteriophages in the treatment of Superbugs?
Lytic life cycle of bacteriophages
The life cycle of bacteriophages has been a good model for understanding how other viruses affect the cells they infect since similar processes have been observed for eukaryotic viruses, which can cause immediate death of the cell or establish a latent or chronic infection. Virulent phages typically lead to the death of the cell through cell lysis (Fig. 7.3). Temperate phages, on the other hand, can become part of a host chromosome and are replicated with the cell genome until such time as they are induced to make newly assembled viruses, or progeny viruses.
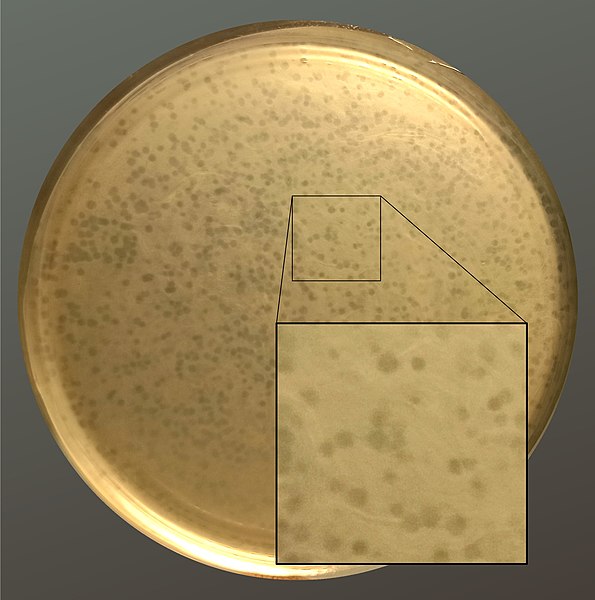
Figure 7.3 Plate showing bacteriophage plaques (clearings) on a dense lawn of the bacterium, Enterococcus faecalis. The clear plaques are where E. faecalis had been lysed. CC-SA-4.0 from Pchelin.
During the lytic cycle, the bacteriophage takes over the cell biosynthetic machinery, reproduces new phages, and destroys the cell. Fig. 7.4 shows the five stages in the bacteriophage lytic cycle.
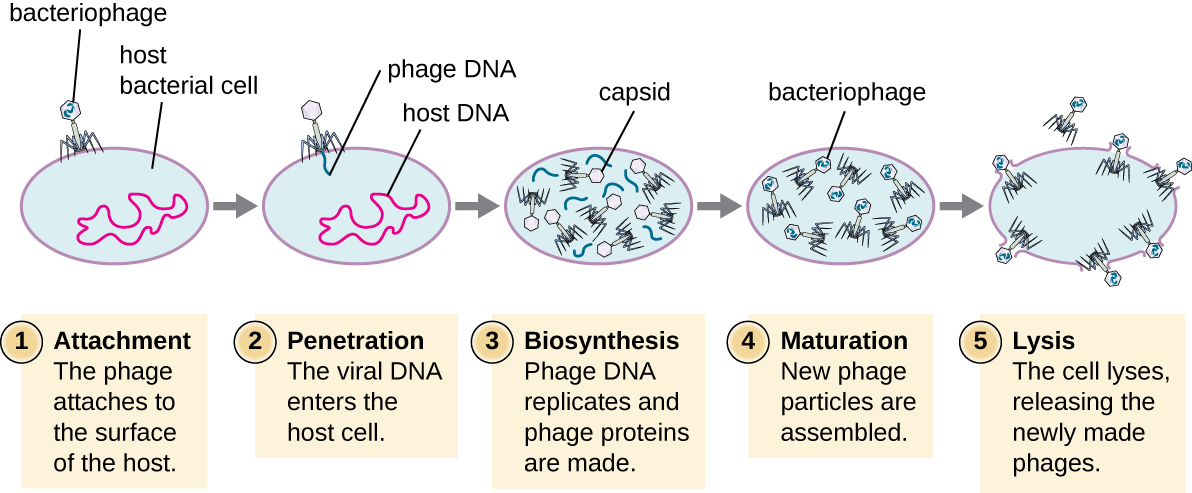
Figure 7.4 Stages of the lytic cycle of a virulent phage. CC-SA-4.0 by Parker.
- The first stage in the infection process is attachment in which the phage interacts with specific surface receptors (e.g., lipopolysaccharides and OmpC protein on host surfaces) on a susceptible host. Most phages have narrow host ranges and may infect one species of bacteria or one strain within a species. This unique recognition property can be exploited for targeted treatment of bacterial infection by phage therapy or for phage typing to identify bacterial strains.
- The second stage of infection is entry or penetration where the viral DNA is injected into and enters the host cell. This occurs through contraction of the tail sheath, which acts like a hypodermic needle to inject the viral genome through the cell wall and membrane. The proteinaceous phage head and remaining components remain outside the bacteria.
- The third stage of infection is biosynthesis of new viral components. After entering the host cell, the virus synthesizes virus-encoded endonucleases to degrade the bacterial chromosome. It then hijacks the host cell to replicate, transcribe, and translate the necessary viral components (capsomeres, sheath, base plates, tail fibers, and viral enzymes) for the assembly of new viruses. Polymerase genes are usually expressed early in the cycle, while capsid and tail proteins are expressed later.
- The fourth stage is the maturation phase, when the viral genomes are packaged into capsid proteins to assemble new phage particles inside the host cells.
- The fifth and final stage is lysis of the host cell and release of the new virions. To liberate free phages, the bacterial cell wall is disrupted by phage proteins such as holin, endolysin, or lysozyme. Mature viruses burst out of the host cell in a process called lysis and the progeny viruses are liberated into the environment to infect new cells.
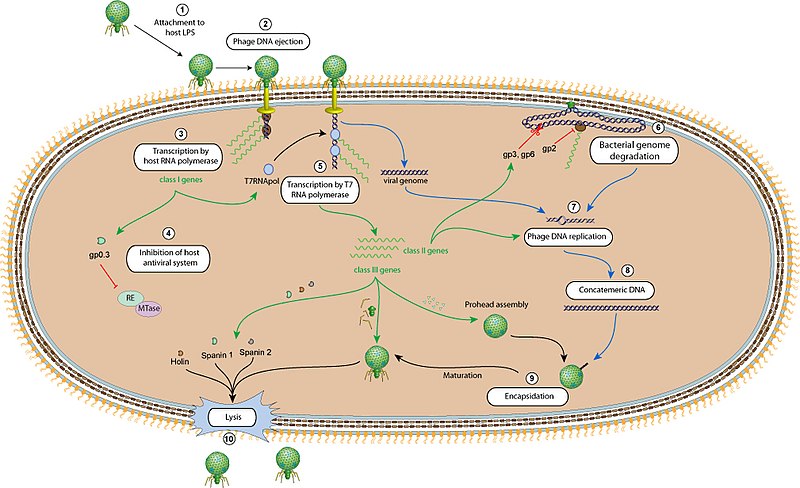
Figure 7.5 Bacteriophage T7 life cycle. CC-SA-4.0 ViralZone.
Fig. 7.5 provides details on the sequence of events taking place inside an infected bacteria during T7 infection. Phage attachment (1) and injection of its DNA (2) are followed by expression of the phage genes. Phage genes are expressed sequentially and divided into early, middle and late genes (Table 7.1). Upon entry into the host cytoplasm, early genes are transcribed by the E. coli RNA polymerase (3). Early genes include the T7 promoter-specific RNA polymerase and a kinase which later inactivates the E. coli RNA polymerase (4), leaving only the T7 RNA polymerase to transcribe T7 middle and late genes (5). The middle genes code for nucleases that digest the E. coli genome (6), an anti-E. coli RNA polymerase, and T7 DNA polymerase to replicate the T7 DNA (7,8). T7 lysozyme later inactivates T7 RNA polymerase and the E. coli apparatus is then focused on translating T7 genes which codes for the T7 core, tail, and capsid proteins that then proceeds to encapsidate the T7 DNA (9). Following assembly of the new virions, the host cell is lyzed (10)
Table 7.1 List of some T7 genes and their function.
|
T7 genes |
Function |
|
Early genes |
|
|
T7 RNA polymerase |
Transcribe T7 genes |
|
Kinase |
Inactivates E. coli RNA polymerase |
|
Anti-restriction enzyme |
Protects T7 DNA by host restriction enzymes |
|
Middle genes |
|
|
Exonuclease |
Degrades E. coli DNA |
|
Endonuclease |
Degrades E. coli DNA |
|
T7 DNA polymerase |
Replicates T7 DNA |
|
Lysozyme |
Inactivates T7 RNA polymerase; degrades peptidoglycan |
|
Late genes |
|
|
Core proteins |
Virion assembly |
|
Tail proteins |
Virion assembly |
|
Capsid proteins |
Virion assembly |
|
Holin |
E. coli lysis |
|
Spannins |
Virion release |
|
|
|
The Lysogenic Cycle
In a lysogenic cycle, the phage genome also enters the cell through attachment and penetration. A prime example of a phage with this type of life cycle is the lambda (l) phage. During the lysogenic cycle, instead of killing the host, the phage genome integrates into the bacterial chromosome and becomes part of the host. The integrated phage genome is called a prophage. A bacterial host with a prophage is called a lysogen. The process in which a bacterium is infected by a temperate phage is called lysogeny. It is typical of temperate phages to be latent or inactive within the cell. As the bacterium replicates its chromosome, it also replicates the phage’s DNA and passes it on to new daughter cells. The presence of the phage may alter the phenotype of the bacterium, since it can bring in extra genes (e.g., toxin genes that can increase bacterial virulence). This change in the host phenotype is called lysogenic conversion or phage conversion. Some bacteria, such as Vibrio cholerae and Clostridium botulinum, are less virulent in the absence of the prophage. In the case of V. cholera, phage encoded toxin can cause severe diarrhea; in C. botulinum, the toxin can cause paralysis. During lysogeny, the prophage will persist in the host chromosome until induction, which results in the excision of the viral genome from the host chromosome. Excision of the phage and induction of the lytic cycle can be triggered by environmental stressors such as starvation or exposure to toxic chemicals.

Figure 7.6 The steps of the lytic and lysogenic cycles. In step 1, the phage attaches outside of a cell then injects DNA into the cell. In step 2, the phage DNA becomes inserted into the host genome to become a prophage. Step 3, the cell divides and prophage DNA is passed to the daughter cells. The image shows the cell dividing and the viral DNA within the host genome also being passed to the daughter cell. In step 4, the viral DNA is excised out of the host genome and the phage enters the lytic cycle. Step 5, phage DNA replicates and phage proteins are made. Step 6, new viral particles are made within the cell. Step 7 is where the bacteria is lyzed and the new phage particles are released. CC-SA-4.0 by Parker
The lambda phage is a temperate phage that can reproduce during the lytic phase or develop a lysogenic phase. During lysogeny, most lambda genes are suppressed, except those required for maintenance of lysogeny. The phages can remain quiescent for thousands of years with immunity to phage infection as a sign that a prophage is present.
During lysogeny, the lambda repressor, cI dimerizes and binds to operator regions (OR and OL) in the right (pR) and left (pL) promoter regions to block binding of RNA polymerase and suppress expression of genes required for phage DNA replication and induction of the lytic phase (Fig. 7.7). In the presence of extensive DNA damage, cI associates with RecA-nucleoprotein, autocleaves, and detaches from the pR and pL operator regions. This allows expression of lytic genes driven by pR and pL, and of the Cro repressor which blocks further synthesis of cI.
The lytic cycle is favored 99% of the time. In rich media where the ratio of phage particles to bacterial counts is low (e.g. low multiplicity of infection, or MOI), 99% of infected cells are lyzed, while at high MOIs, around half of infected cells form lysogens.
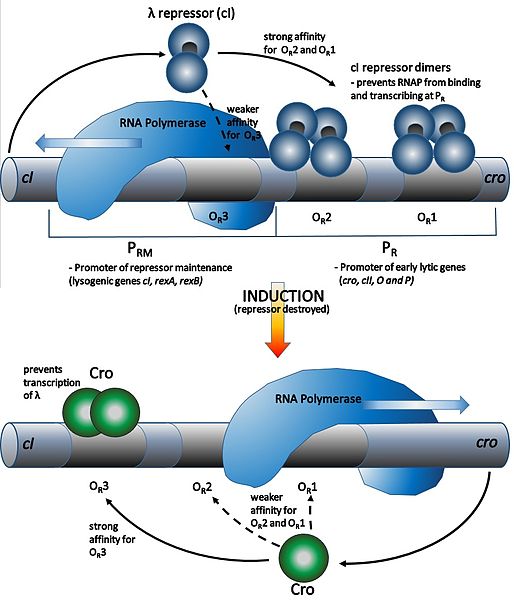
Figure 7.7 Lysogeny and lysis induction in Lambda bacteriophage. In the lysogenic phase (upper figure), the repressor protein, cI, binds to the operator (OR) of the right promoter, PR, to block expression of lytic genes. In the lytic phase, cI detaches from OR and RNA polymerase is able to bind to PR and transcribe the lytic genes. CC-AS-3.0 from Chimb
Cosmids
Lambda bacteriophage has linear DNA which circularizes when free in the E. coli cytoplasm. Circularization is due to the presence of 12 complementary base pairs in a 200 bp cohesive or cos site. The phage terminase nicks at the cos sites of concatameric DNA to package one linear genome per head. Insertion of the cos site into any fragment of DNA facilitates packaging of the DNA into l phage heads. This property was applied in the design of cosmids (Fig. 7.8) – plasmids with cos sites which can get packaged into phage heads, and which can transduce DNA into E. coli where the cosmids then circularizes. Cosmids are useful in the construction of genomic libraries for use in whole genome sequencing and for complementation experiments.

Figure 7.8 Cosmids are plasmids with l cos sites. The cos sites are nicked by l terminase (inverted triangles) during genome encapsidation in the phage head. During infection, the injected linear DNA circularizes in the bacterial cytoplasm similar to plasmids. CCO 1.0 by Zlir’a.
Recombineering
The l Red recombineering system (Fig. 7.9) makes use of l phage recombination proteins to perform insertions and/or deletions in E. coli (Fig. 7.10). Like RecB, l exonuclease (Exo) removes bases in a 5’-3’ direction of one DNA strand. The remaining single DNA strand is coated with Beta and the resulting Beta-nucleoprotein invades and recombines with homologous sequences. Gam serves to inhibits the activity of E. coli RecBCD and protects the transformed DNA from degradation by RecB.
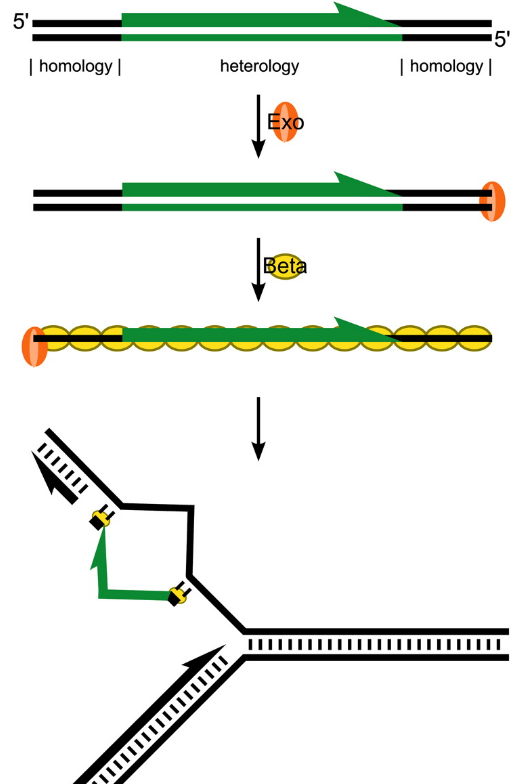
Figure 7.9. Lambda Red recombineering. Exo removes bases in a 5’-3’ direction of one DNA strand. The remaining single stranded DNA is coated with Beta and the resulting Beta-nucleoprotein invades and recombines with homologous sequences.
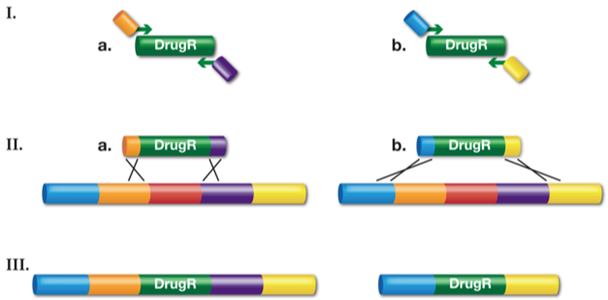
Figure 7.10. Recombineering of dsDNA to generate gene replacement knockouts and deletions. I.) A drug-resistant cassette is made by PCR using two, ~70-base hybrid primers. The 5’ ends of the primers have homology to the desired target (~50-base) and the 3’ end of the primers (~20-base) have sequences complementary to the drug-resistance gene (DrugR). II.) The linear drug-resistant cassette made by PCR is transformed into recombination-competent cells and Red-mediated recombination occurs. Depending on what PCR product is used, (a) or (b), the final recombinant will be a gene swap (IIIa) or a more substantial deletion (IIIb) respectively. Adapted from National Cancer Institute.
Acute and chronic infections
Infections which lead to host lysis and release of new virions can be described as acute infections. In archaeal viruses and filamentous phages, there is a less common chronic type of infection where the viral genome does not integrate into the host chromosome and phages slowly bud off from the host over time without lysing the host. Acute infections occur in both Gram negative and Gram positive bacteria while chronic infections are only in Gram negative bacteria.
7.2 Transduction
Transduction occurs when a bacteriophage transfers bacterial DNA from one bacterium to another during sequential infections. Transduction plays an important role in the evolutionary process of bacteria, giving them a mechanism for asexual exchange of genetic information. There are two types of transduction: generalized and specialized. During the lytic cycle of viral replication, the virus hijacks the host cell, degrades the host chromosome, and makes more viral genomes. As it assembles and packages DNA into the phage head, packaging occasionally makes a mistake. Instead of packaging viral DNA, it inserts a random piece of host DNA inside the capsid. This virion can then then inject the former host’s DNA into a newly infected host. The asexual transfer of genetic information can allow for DNA recombination to occur, thus providing the new host with new genes (e.g., an antibiotic-resistance gene, virulence genes, or a sugar-metabolizing gene). Generalized transduction occurs when a random piece of bacterial chromosomal DNA is captured and transferred by a phage during the lytic cycle; it occurs with non-integrating phages. Because random pieces of the host DNA can be transduced, generalized transduction is useful for transfer of genes, mutations, or traits from a donor host to a new recipient for strain engineering. Generalized transduction is also useful for complementation and marker rescue studies.
Specialized transduction happens with integrating phages and occurs at the end of the lysogenic cycle. Some conditions (e.g., ultraviolet light or chemical exposure) stimulate the prophage to undergo induction, causing the phage to excise from the genome, enter the lytic cycle, and produce new phages. During the process of prophage excision from the host chromosome, the phage may occasionally remove some bacterial DNA near the site of viral integration. The phage and host DNA from one end or both ends of the integration site are packaged within the capsid and are transferred to the new, infected host. Since the DNA transferred by the phage is not randomly packaged but is instead a specific piece of DNA near the site of integration, this mechanism of gene transfer is referred to as specialized transduction (Fig. 7.11). The DNA can then recombine with host chromosome, giving the recipient host new characteristics.
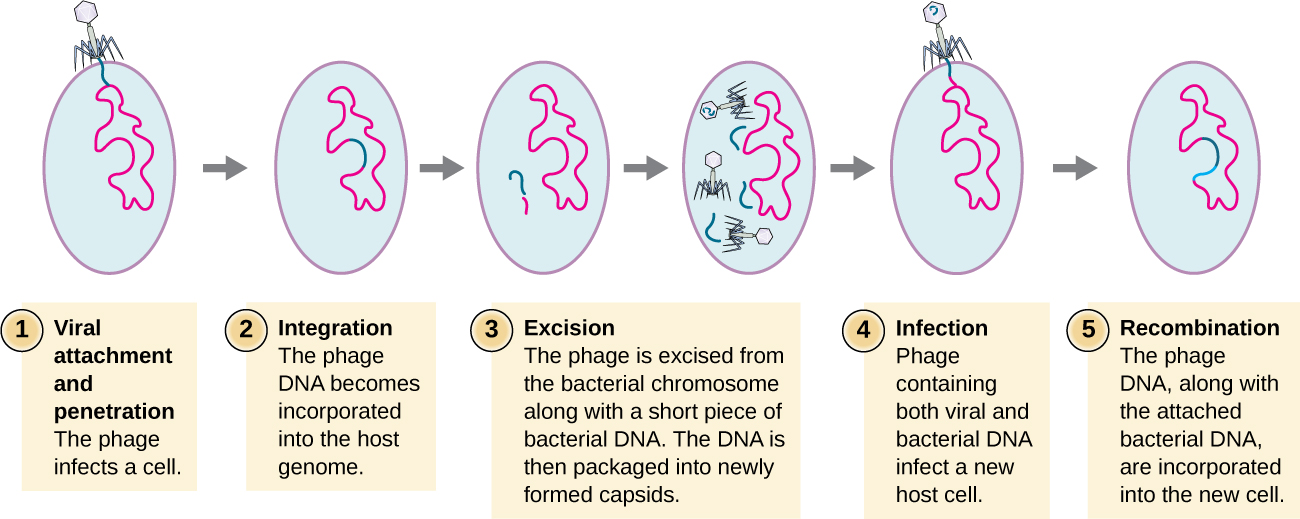
Figure 7.11. Steps of specialized transduction. Step 1 – viral attachment and penetration. Step 2 -integration of the phage DNA (blue) into the host genome (red). Step 3 – excision where phage DNA is excised from the bacterial chromosomes along with a short piece of host DNA adjacent to the insertion point. The bacteria/phage DNA is then packaged into newly formed capsids. Step 4 is infection of a new host cell by a phage carrying bacteria/phage DNA. Step 5 is recombination when the phage DNA along with the attached bacterial DNA integrates into the genome of the new host. The image shows a new bacterial cell with virus DNA as well as other bacterial DNA in its genome. CC-SA-4.0 by Parker.
Lysogeny and pathogenic conversion
Many pathogenic bacteria are able to produce toxins, biological poisons that assist in their ability to invade and cause damage to tissues. Toxins can be categorized as endotoxins or exotoxins. The lipopolysaccharide (LPS) found on the outer membrane of Gram-negative bacteria is called endotoxin. During infection and disease, Gram-negative bacteria release endotoxins when the cell dies or when the bacterium undergoes binary fission. The lipid component of endotoxin, lipid A, is primarily responsible for the toxic properties of the LPS. Lipid A is relatively conserved across different genera of Gram-negative bacteria; therefore, the toxic properties of lipid A are similar regardless of the Gram-negative pathogen. High concentrations of endotoxin in the blood can cause an excessive inflammatory response, leading to a severe drop in blood pressure, multi-organ failure, and death.
Unlike endotoxins, exotoxins are protein molecules that are produced by a wide variety of living pathogenic bacteria. Some Gram-negative pathogens produce exotoxins, but the majority are produced by Gram-positive pathogens. Exotoxins differ from endotoxin in several key characteristics, summarized in Table 7.2. In contrast to endotoxin, which stimulates a general systemic inflammatory response when released, exotoxins are much more specific in their actions and the cells they interact with. Each exotoxin targets specific receptors on specific cells and damages those cells through unique molecular mechanisms. Endotoxin remains stable at high temperatures and requires heating at 121 °C (250 °F) for 45 minutes to inactivate. By contrast, most exotoxins are heat labile because of their protein structure, and many are denatured (inactivated) at temperatures above 41 °C (106 °F). As discussed earlier, endotoxin can stimulate a lethal inflammatory response at very high concentrations and has a measured LD50 of 0.24 mg/kg. By contrast, very small concentrations of exotoxins can be lethal. For example, botulinum toxin, which causes botulism, has an LD50 of 0.000001 mg/kg (240,000 times more lethal than endotoxin).
Table 7.2. Comparison of endotoxins and exotoxins produced by bacteria. Adapted from Parker et al. CC-SA-4.0
|
|
Endotoxin |
Exotoxin |
|
Source
|
Gram-negative bacteria |
Gram-positive and Gram-negative bacteria |
|
Composition |
Lipid A component of lipopolysaccharide |
Protein |
|
Effects on host |
Systemic inflammation and fever |
Specific damage to certain cells |
|
LD50 |
High |
Low |
|
Heat stability |
Heat stable |
Most are heat labile |
|
Toxoids |
Cannot be made |
Can be made using formalin |
|
|
|
|
Exotoxins cause disease or cell death by three mechanisms: intracellular targeting, membrane disrupting, and superantigens. Many exotoxins are encoded by bacteriophages (Table 7.3). Bacteria without phage sequences tend to be less pathogenic.
Table 7.3 Phage encoded exotoxins, the bacteria that produces them, and the diseases caused by the toxins. Adapted from Parker, et al. and Boyd and Brussow (1).
|
Host bacteria |
Exotoxin |
Disease |
Mechanism |
|
Escherichia coli 0157:H7 |
Shiga toxin |
Dysentery |
Inactivates 28SrRNA |
|
Staphylococcus aureus |
Toxic shock syndrome toxin |
Toxic shock syndrome |
Excessive activation of immune system cells and release of cytokines (chemical mediators) from immune system cells. Life-threatening fever, inflammation, and shock are the result. |
|
Vibrio cholerae |
Cholera toxin |
Cholera |
Activation of adenylate cyclase in intestinal cells, causing increased levels of cyclic adenosine monophosphate (cAMP) and secretion of fluids and electrolytes out of cell, causing diarrhea
|
|
Clostridium botulinum |
Botulinum toxin |
Botulism |
Inhibits release of the neurotransmitter acetylcholine from neurons, resulting in flaccid paralysis |
|
Corynebacterium diphtheriae |
Diphtheria toxin |
Diphtheria |
Inhibition of protein synthesis, causing cellular death |
|
Pseudomonas aeruginosa |
Cytotoxin |
Cytotoxicity |
|
|
Streptococcus pyogenes |
Toxin A and C |
Scarlet fever |
|
Shiga toxin
Shiga toxin (Stx) is produced by E. coli O157:H7. E. coli O157:H7 harbors more than 18 prophage-like elements which account for more than 50% of DNA not found in the chromosome of other strains of E. coli. The toxin causes dysentery which is characterized by watery to bloody diarrhea and can lead to acute kidney failure and damage to the central nervous system. Dysentery is a major cause of infant mortality worldwide.
Shiga toxin belongs to the A-B type toxins: StxA has enzymatic activity and StxB functions for binding. Stx B subunit attaches to a receptor found on the cell membrane of target cells and facilitates endocytosis of the toxin into cells. In the vacuole, StxA is separated from B and released into the cytoplasm. In the cytoplasm, StxA has N-glycosylase that removes removes a single adenine from 28s rRNA (peptidyl transferase) thus inactivating 28s rRNA and stopping translation and leading to cell death.
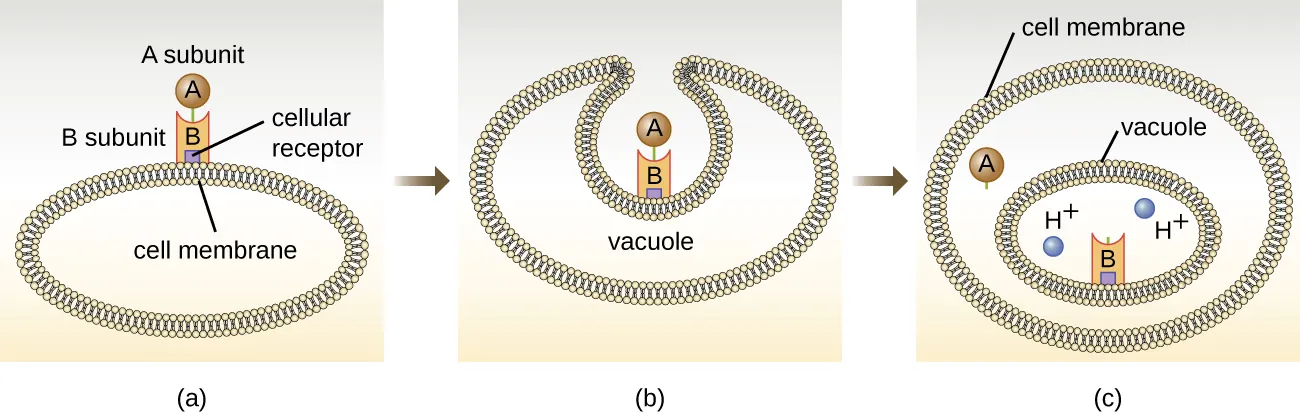
Figure 7.12 (a) In A-B toxins, the B component binds to the host cell through its interaction with specific cell surface receptors. (b) The toxin is brought into the host cell through endocytosis. (c) Once inside the vacuole, the A component (active component) separates from the B component and the A component gains access to the cytoplasm and to its target. CC-SA-4.0 by Parker
Other examples of A-B toxins are the diphtheria, cholera, botulinum, and tetanus toxins. The diphtheria toxin (Dtx) is produced by the Gram-positive bacterium, Corynebacterium diphtheriae, the causative agent of nasopharyngeal and cutaneous diphtheria. After the A subunit of Dtx (DtxA) separates and gains access to the host cytoplasm, DtxA facilitates the transfer of adenosine diphosphate (ADP)-ribose onto an elongation-factor (EF-2) that is needed for protein synthesis. Hence, diphtheria toxin inhibits protein synthesis in the host cell, ultimately killing the cell (Fig. 7.13).
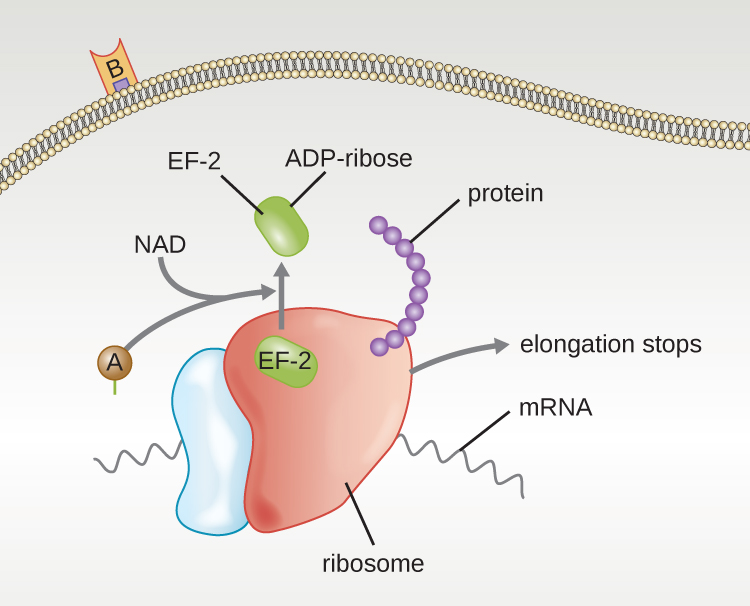
Figure 7.13 Diphtheria toxin inhibition of protein synthesis. The A subunit inactivates EF2 by attachment of an ADP-ribose. This stops protein elongation, inhibiting protein synthesis, and killing the cell. CC-SA-4.0 by Parker.
Cholera toxin is produced by the Gram-negative bacterium Vibrio cholerae and is composed of one A subunit and five B subunits. The B subunits bind to receptors on the intestinal epithelial cells of the small intestines. After gaining entry into the cytoplasm of the epithelial cell, the A subunit ADP-ribosylates an intracellular G protein. The activated G protein, in turn, leads to the activation of the enzyme adenyl cyclase, which begins to produce more cyclic AMP. The increased cAMP causes intestinal cells to secrete excessive amounts of water and NaCl into the lumen of the intestinal tract, resulting in severe diarrhea.
Watch how the cholera toxin affects cell function:
A third class of exotoxins is the superantigens. These are exotoxins that trigger an excessive, nonspecific stimulation of immune cells to secrete cytokines (chemical messengers). The excessive production of cytokines, often called a cytokine storm, elicits a strong immune and inflammatory response that can cause life-threatening high fevers, low blood pressure, multi-organ failure, shock, and death. The prototype superantigen is the toxic shock syndrome toxin of S. aureus. The toxin binds to proteins on the surface of T cells, leading to cytokine storm which causes immune cells to attack multiple organs. Most toxic shock syndrome cases are associated with vaginal colonization by toxin-producing S. aureus in menstruating women; however, colonization of other body sites can also occur. The TSS genes are found in pathogenicity islands that get expressed only in the presence of helper phage. Phage genes in the islands are packaged into helper phage heads that can infect other bacteria, where the phage sequences can then integrate into the genome of the newly infected host.
7.3 Bacteria defenses against bacteriophages
Bacteria have evolved defenses to protect against bacteriophage infection.
- Loss or decreased expression of the phage receptor. Since adsorption of the phage onto the surface of a bacterial cell is the first step in successful infection, loss or decreased expression of the receptor protects the bacteria from phage infection. While the mutations increase resistance to phage infection, the same mutations can lead to decreased fitness or virulence. Some bacteria have mechanisms also that block phage access to the receptors (3).
- Restriction-modification systems. Many bacteria have evolved to produce restriction enzymes that are able to recognize and cleave injected phage DNA. Some phages, in response, contain modified bases (Ex. T4 hydroxymethycytosine) that the restriction enzymes are not able to recognize or cut. To counter resistant phage DNA, bacteria in turn have evolve enzymes that are able to recognize and cut through the modified bases (3).
- Abortive infection (Abi) is where an infected cell commits suicide to contain the injected viral genome inside its cytoplasm, prevent phage replication, and stop the spread of infection (2).
- CRISPR-CAS. Clustered regularly interspaced short palindromic repeats (CRISPRs) and CRISPR-associated (CAS) genes. CRISPR-CAS system of bacteria makes use of guide RNA to bind and target phage DNA for cleavage by the CAS endonuclease (2).
7.4 Bacteriophage use in medicine
Several companies are now exploring the use of bacteriophages for controlling and treating bacterial infections both in animals and humans. The bacteria targeted by bacteriophages and diseases that they cause are listed in Table 7.4
Table 7.4. Bacteria targeted by phage therapy.
|
Bacteria |
Disease |
|
Pseudomonas aeruginosa |
Lung infections |
|
Staphylococcus aureus |
Bacteremia and prosthesis infections |
|
Klebsiella pneumoniae |
Pneumonia |
|
Escherichia coli O157:H7 |
Food poisoning |
|
Listeria monocytogenes |
Food poisoning |
|
Salmonella spp |
Food poisoning |
|
Shigella spp |
Food poisoning |
|
Campylobacter jejuni |
Food poisoning |
Watch:
Adapted and remixed from Parker et al. CC-SA-4.0
Access for free at https://openstax.org/books/microbiology/pages/1-introduction
References:
- Boyd EF, Brüssow H. Common themes among bacteriophage-encoded virulence factors and diversity among the bacteriophages involved. Trends Microbiol. 2002;10(11):521-529. doi:10.1016/s0966-842x(02)02459-9.
- González-Delgado A, Mestre MR, Martínez-Abarca F, Toro N. Prokaryotic reverse transcriptases: from retroelements to specialized defense systems. FEMS Microbiol Rev. 2021 Nov 23;45(6):fuab025. doi: 10.1093/femsre/fuab025. PMID: 33983378; PMCID: PMC8632793.
- Labrie, S., Samson, J. & Moineau, S. Bacteriophage resistance mechanisms. Nat Rev Microbiol 8, 317–327 (2010). https://doi.org/10.1038/nrmicro2315
End-of-Chapter Questions:

